The Fabry Disease Market is estimated to be valued at USD 2.4 billion in 2025 and is projected to reach USD 5.2 billion by 2035, registering a compound annual growth rate (CAGR) of 8.0% over the forecast period.
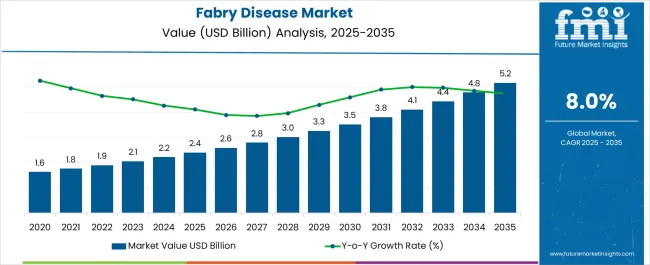
| Metric | Value |
|---|---|
| Fabry Disease Market Estimated Value in (2025 E) | USD 2.4 billion |
| Fabry Disease Market Forecast Value in (2035 F) | USD 5.2 billion |
| Forecast CAGR (2025 to 2035) | 8.0% |
The Fabry disease market is witnessing steady expansion, supported by rising diagnosis rates, improved access to specialty treatments, and continuous advancements in rare disease therapeutics. Enzyme replacement therapy and emerging modalities are shaping the treatment landscape as more patients are being identified through newborn screening programs and genetic testing initiatives. Governments and healthcare organizations are increasingly focused on improving awareness and patient registries, which is helping to address the historic challenge of underdiagnosis.
Pharmaceutical companies are investing heavily in clinical research and targeted therapies, aiming to improve long term outcomes and quality of life for patients. The market is further supported by favorable regulatory frameworks and orphan drug incentives that encourage the development of new treatment options.
Increased collaboration between healthcare providers, patient advocacy groups, and research institutions is also driving improvements in treatment access and affordability As more innovative therapies enter the pipeline, including next generation enzyme replacement and gene therapies, the market outlook remains positive, with sustained growth expected in both established and emerging healthcare systems worldwide.
The fabry disease market is segmented by treatment, end user type, and geographic regions. By treatment, fabry disease market is divided into Enzyme replacement therapy, Gene therapy, Pharmaceutical formulations containing agalsidase alpha, Analgesics, Anticonvulsants, and NSAIDs. In terms of end user type, fabry disease market is classified into Hospitals and Clinics. Regionally, the fabry disease industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
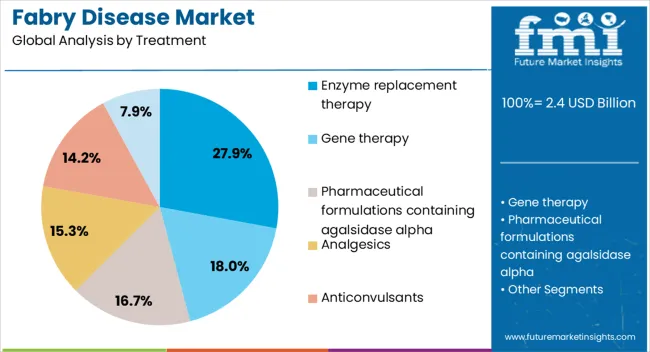
The enzyme replacement therapy segment is projected to hold 27.9% of the Fabry disease market revenue share in 2025, making it one of the leading treatment approaches. This position is being reinforced by its established clinical efficacy in reducing the buildup of globotriaosylceramide and improving organ function in patients with Fabry disease. The availability of recombinant enzyme formulations that target the underlying enzyme deficiency has provided a cornerstone therapy option for many patients globally.
Despite the emergence of newer modalities, enzyme replacement therapy continues to be widely adopted due to its proven safety record, regulatory approvals across multiple regions, and well established clinical use. Increased patient access through expanded reimbursement programs and government supported initiatives is further strengthening its uptake.
The therapy’s ability to slow disease progression and reduce complications in renal, cardiac, and neurological functions has ensured continued relevance Ongoing research into improved formulations with enhanced stability and reduced immunogenicity is also expected to sustain its role as a vital treatment segment in the coming years.
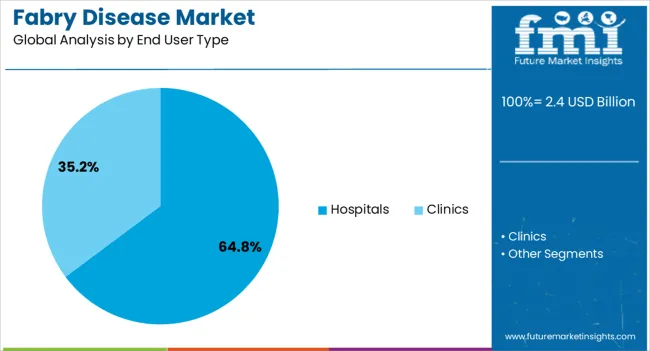
The hospitals segment is expected to account for 64.8% of the Fabry disease market revenue share in 2025, positioning it as the dominant end user type. This dominance is being driven by the specialized nature of Fabry disease management, which requires advanced diagnostic tools, multidisciplinary teams, and infusion facilities typically concentrated in hospital settings. Hospitals are the primary point of care for enzyme replacement therapy and emerging advanced treatments, which often require close monitoring and regular administration.
The presence of specialized centers of excellence within hospitals has further strengthened their role in delivering comprehensive Fabry disease care, from diagnosis to long term management. Growing investments in rare disease clinics and the integration of genetic counseling services within hospital systems are improving patient access and treatment outcomes.
Furthermore, hospitals are actively involved in clinical research and trials, enabling early access to innovative therapies and driving adoption of new treatment modalities As global healthcare infrastructure strengthens and rare disease awareness improves, hospitals are expected to maintain their leading position in providing Fabry disease care.
The market for Fabry disease is predicted to expand over the forecast period owing to the continued increase in the rate of incidences of this genetic disease. Generally, therapeutic approaches such as enzyme replacement therapy (ERT) and chaperone, developed to improve clinical symptoms of patients suffering from Fabry disease, are used to treat the patients. Consequently, an increasing number of patients and a rise in the use of these treatments are predicted to facilitate market development over the projected period. Furthermore, the increasing prevalence of late-onset and type 1 classic Fabry disease, especially among males, is contributing to the overall market growth.
A recent study indicates that Fabry disease is emerging as one of the leading genetic metabolic storage diseases. The disease often results in progressive organ impairment or failure, or premature death. Additionally, Fabry disease patients aged 40 years or beyond are susceptible to heart problems, which cause abnormal expansion of heart muscles due to additional effort needed to pump blood. Thus, an increase in the aging population is predicted to boost the diagnosis and treatment of Fabry disease, further expanding the Fabry disease market over the forecast period.
The market is also stimulated by the rising organ-related problems usually found in patients with late-onset of Fabry disease. Various other factors contributing to market development over the forecast period include:
The global Fabry disease market growth might impede owing to the financial burden of patients suffering from rare genetic disorders, such as Fabry, and the huge costs associated with its treatment. For instance, the overall expenditure of enzyme replacement therapy (ERT) can rise up to US$ 200,000 or beyond each year. In addition to this, the high costs of treatment offered by major patient-dedicated biotechnology companies might restrict the market growth. For instance, Amicus Therapeutics Inc. offers oral chaperone therapy named Galafold (migalastat), which is priced at US$ 315,000 per year. All these factors are likely to hinder market development going forward.
The major drivers that drive the fabry disease market growth are extensive R&D activities, adoption of advanced technologies in the research practices. Due to hereditary nature and severity of the disease, complications in physiological systems, such as cardiac system, urinary system and others are expected to increase the demand for trustworthy treatment. Extensive R&D practices is one of the valuable drivers of the market.
Due to this, various therapeutic products, such as, PRX-102, JR-051, NP-003, GC-1119 and others, are under pipeline studies. However, rare incidence rate (1 in 40,000), lack of awareness about advancements in genetic sciences, technological limitations in certain region of the world are some of the restraining factors that may affect the growth of the fabry disease market.
The enzyme replacement therapy is designed to provide enzyme to the patients suffering from enzyme deficiency. It was found that this treatment was not a complete cure though it improved the metabolism.
Enzyme replacement therapy is the most expensive and unaffordable treatment among the treatments for fabry disease, hence the market for enzyme replacement therapy is expected to register moderate growth in overall fabry disease market. The analgesics, anticonvulsants and NSAIDs are generally known for the treatment of pain and other symptoms associated with fabry disease hence, contributing majorly to the market.
Geographically, the global fabry disease market is classified into regions namely, North America, Latin America, Western Europe, Eastern Europe, APAC, Japan, Middle East and Africa.North America is estimated to be the most lucrative region owing to the affordability of the treatment followed by Europe. The rare incidences in APAC and MEA countries are expected to limit the growth of fabry disease market in these regions.
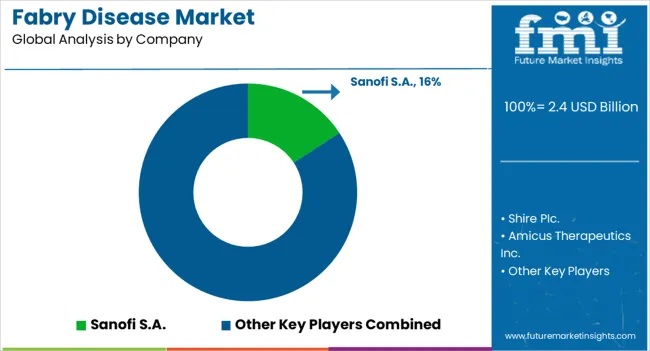
The major players operating in this market and involved in development of new medications include Sanofi-Aventis LLC, iBio, Inc., GlaxoSmithKline plc, Genzyme Corporation, and Neuraltus Pharmaceuticals, Inc. Some of the existing key players in the global fabry disease market are Novartis Pharmaceuticals, Merc & Co., Inc., Bristol-Myers Squibb Company, AbbVie Inc., Amgen Inc., Teva pharmaceutical Industries Ltd., Pfizer Inc., Takeda Pharmaceutical Co. Ltd.
The report is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain. The report provides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
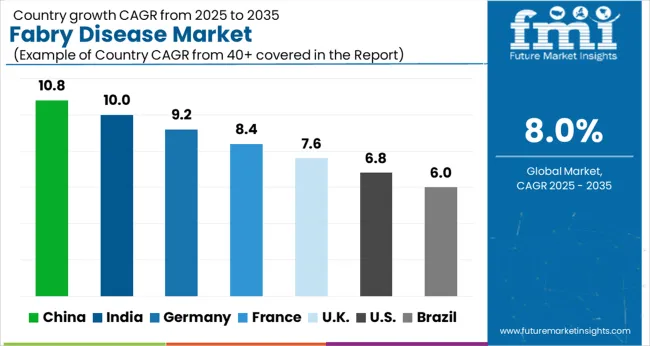
| Country | CAGR |
|---|---|
| China | 10.8% |
| India | 10.0% |
| Germany | 9.2% |
| France | 8.4% |
| UK | 7.6% |
| USA | 6.8% |
| Brazil | 6.0% |
The Fabry Disease Market is expected to register a CAGR of 8.0% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 10.8%, followed by India at 10.0%. Developed markets such as Germany, France, and the UKcontinue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 6.0%, yet still underscores a broadly positive trajectory for the global Fabry Disease Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 9.2%. The USA Fabry Disease Market is estimated to be valued at USD 883.2 million in 2025 and is anticipated to reach a valuation of USD 1.7 billion by 2035. Sales are projected to rise at a CAGR of 6.8% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 114.2 million and USD 75.9 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 2.4 Billion |
| Treatment | Enzyme replacement therapy, Gene therapy, Pharmaceutical formulations containing agalsidase alpha, Analgesics, Anticonvulsants, and NSAIDs |
| End User Type | Hospitals and Clinics |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
| Key Companies Profiled | Sanofi S.A., Shire PIc., Amicus Therapeutics Inc., ISU Abxis Co Ltd., JCR Pharmaceuticals Co Ltd., Protalix Biotherapeutics Inc., Idorsia Pharmaceuticals Ltd., Avrobio Inc., Takeda Pharmkceutical Co Ltd., Chiesi Farmaceutici SpA, Freeline Therapeutics Holdings PLC, Yuhan Corp, and MOP Therapeutics |
The global fabry disease market is estimated to be valued at USD 2.4 billion in 2025.
The market size for the fabry disease market is projected to reach USD 5.2 billion by 2035.
The fabry disease market is expected to grow at a 8.0% CAGR between 2025 and 2035.
The key product types in fabry disease market are enzyme replacement therapy, gene therapy, pharmaceutical formulations containing agalsidase alpha, analgesics, anticonvulsants and nsaids.
In terms of end user type, hospitals segment to command 64.8% share in the fabry disease market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Disease Resistant Mask Market Analysis - By Type, Material, End-User, Distribution Channel, and Region - Trends, Growth & Forecast 2025 to 2035
Rare Disease Clinical Trials Market Size and Share Forecast Outlook 2025 to 2035
The lung disease therapeutics market is segmented by disease type, treatment type and distribution channel from 2025 to 2035
Rare Disease Gene Therapy Market
Swine Disease Diagnostic Kit Market Size and Share Forecast Outlook 2025 to 2035
Liver Disease Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Byler Disease Market
Airway Disease Treatment Market Size and Share Forecast Outlook 2025 to 2035
Celiac Disease Diagnostics Market Analysis - Size, Share & Forecast 2025 to 2035
Shrimps Disease Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Chronic Disease Management Market Size and Share Forecast Outlook 2025 to 2035
The Addison Disease Testing Market Is Segmented by Test Type, and End User from 2025 To 2035
Pleural Diseases Therapeutics Market – Drug Trends & Future Outlook 2025 to 2035
Crohn’s Disease (CD) Treatment Market Analysis & Forecast by Drug Type, Distribution Channel and Region through 2035
Sandhoff Disease Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Zoonotic Disease Treatment Market Size and Share Forecast Outlook 2025 to 2035
Wilson’s Disease Diagnostics Market Analysis – Size, Share & Forecast 2023-2033
Infectious Disease Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Predictive Disease Analytics Market Size and Share Forecast Outlook 2025 to 2035
Autoimmune Disease Therapeutics Market Analysis – Size, Share, & Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA