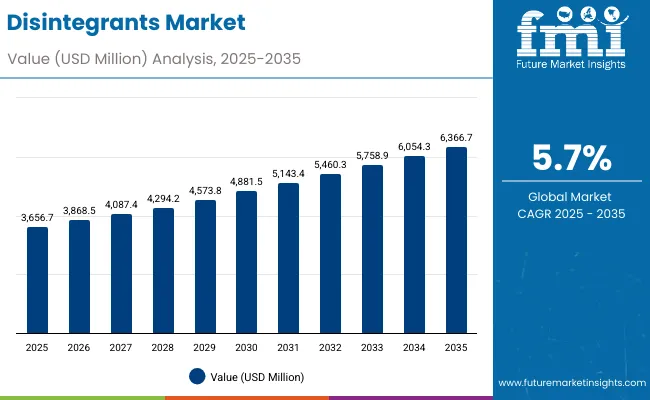
The disintegrants market is valued at USD 3,656.7 million in 2025 and is projected to reach USD 6,366.7 million by 2035, advancing at a CAGR of 5.7%. Growth is driven by increasing demand for advanced pharmaceutical formulations, rising adoption of specialized tablet manufacturing technologies, and growing focus on patient compliance optimization across the global pharmaceutical and nutraceutical sectors. Disintegrants offer optimized drug bioavailability, improved tablet breakdown efficiency, and enhanced therapeutic effectiveness, making them suitable for pharmaceutical companies, contract manufacturing organizations, and nutraceutical production facilities.
Synthetic disintegrants account for the largest share at 60% due to superior performance characteristics, precise disintegration timing, and compatibility with advanced pharmaceutical infrastructure, while natural systems support biocompatible applications and specialized formulation requirements. Pharmaceutical applications represent the dominant share of demand at 70%, followed by nutraceutical programs as manufacturers expand therapeutic optimization offerings.
Disintegrants Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 3,656.7 million |
| Market Forecast Value (2035) | USD 6,366.7 million |
| Forecast CAGR (2025 to 2035) | 5.7% |
Asia Pacific, particularly India and China, leads growth due to pharmaceutical infrastructure expansion and generic drug manufacturing modernization, while Europe and North America show steady demand supported by established pharmaceutical operations. Competition in the disintegrants market remains moderately consolidated, with key companies such as Ashland Inc., DuPont, and JRS Pharma focusing on superdisintegrant technologies, formulation optimization systems, and technical support capabilities to strengthen market positioning.
The pharmaceutical industry's increasing emphasis on patient compliance has transformed disintegrants from simple tablet additives into sophisticated functional ingredients that determine drug bioavailability and therapeutic effectiveness. Modern immediate-release formulations require precise disintegration timing to achieve optimal drug dissolution profiles, particularly for poorly soluble active pharmaceutical ingredients where rapid tablet breakdown is critical for therapeutic efficacy. Advanced superdisintegrants like crospovidone and croscarmellose sodium have revolutionized tablet design by enabling faster disintegration times while maintaining tablet integrity during manufacturing, storage, and handling.
The rise of orodispersible tablets represents a paradigm shift in pharmaceutical delivery systems, driven by aging populations who struggle with swallowing conventional tablets and pediatric applications requiring palatable, easy-to-administer formulations. These specialized dosage forms demand disintegrants that function effectively in minimal saliva volumes while producing acceptable taste profiles, creating technical challenges that have spurred innovation in both natural and synthetic disintegrant technologies. Pharmaceutical companies increasingly rely on specialized disintegrant grades that combine rapid water uptake with controlled swelling properties to achieve optimal disintegration performance.
Generic drug manufacturing has created substantial demand for cost-effective disintegrant solutions that maintain bioequivalence with brand-name products while optimizing production economics. Contract manufacturing organizations face increasing pressure to develop robust formulations that perform consistently across different environmental conditions and raw material variations, making disintegrant selection a critical factor in manufacturing success. The global expansion of generic pharmaceutical production, particularly in emerging markets, has driven demand for reliable, well-characterized disintegrant grades that meet international regulatory standards.
Between 2025 and 2030, the disintegrants market is projected to expand from USD 3,656.7 million to USD 4,881.5 million, resulting in a value increase of USD 1,224.8 million, which represents 45.2% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for advanced pharmaceutical formulations and specialized tablet technologies, product innovation in synthetic disintegrant applications and natural ingredient systems, as well as expanding integration with pharmaceutical manufacturing and nutraceutical applications. Companies are establishing competitive positions through investment in specialized production capabilities, advanced processing technologies, and strategic market expansion across pharmaceutical, nutraceutical, and veterinary medicine applications.
From 2030 to 2035, the market is forecast to grow from USD 4,881.5 million to USD 6,366.7 million, adding another USD 1,485.2 million, which constitutes 54.8% of the overall ten-year expansion. This period is expected to be characterized by the expansion of specialized pharmaceutical applications, including advanced disintegrant-based formulations and next-generation tablet technologies tailored for specific therapeutic requirements, strategic collaborations between disintegrant producers and pharmaceutical companies, and an enhanced focus on quality standards and automated processing protocols. The growing emphasis on pharmaceutical innovation and nutraceutical development will drive demand for comprehensive disintegrant solutions across diverse manufacturing applications.
The disintegrants market grows by enabling pharmaceutical manufacturers and nutraceutical companies to optimize formulation processes while accessing specialized excipient materials without substantial in-house development investment.
Manufacturing companies and pharmaceutical operators face mounting pressure to develop advanced tablet formulations and specialized drug delivery systems while managing complex regulatory requirements, with high-performance synthetic disintegrants typically providing 40-60% performance enhancement compared to conventional alternatives, making advanced superdisintegrant technologies essential for competitive pharmaceutical positioning. The pharmaceutical industry's need for precise disintegration control and application-specific formulation capabilities creates demand for comprehensive disintegrant solutions that can provide superior performance, maintain consistent quality standards, and ensure reliable operation without compromising tablet integrity or therapeutic effectiveness.
Government initiatives promoting advanced pharmaceutical technologies and drug development drive adoption in tablet manufacturing, nutraceutical production, and veterinary medicine applications, where formulation quality has a direct impact on therapeutic performance and long-term market effectiveness. However, supply complexity constraints during large-scale production projects and the expertise requirements for specialized disintegrant integration may limit accessibility among smaller pharmaceutical companies and developing regions with limited technical infrastructure for advanced formulation systems.
The market is segmented by product type, form, application, source, sales channel, functionality, end user, and region. By product type, the market is divided into natural disintegrants and synthetic disintegrants. Based on form, the market is categorized into powder, granules, capsules, and tablets. By application, the market includes pharmaceuticals, nutraceuticals, and veterinary medicine. By source, the market includes plant-based, microbial-based, mineral-based, and synthetic.
By sales channel, the market is divided into B2B and B2C. By functionality, the market includes superdisintegrants and conventional disintegrants. By end user, the market includes pharmaceutical companies, contract manufacturing organizations, and nutraceutical companies. Regionally, the market is divided into Asia Pacific, Europe, North America, and other key regions.
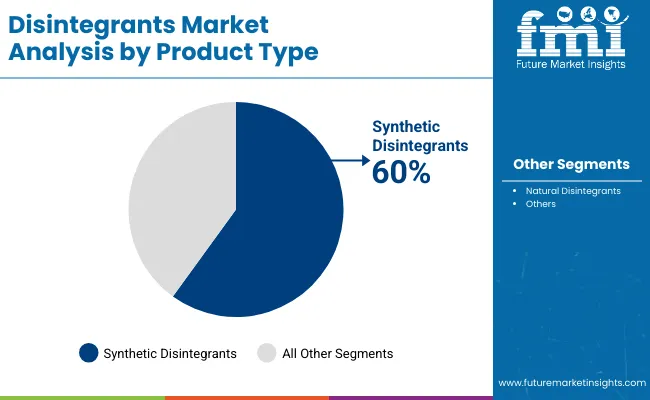
The synthetic disintegrants segment represents the dominant force in the disintegrants market, capturing approximately 60.0% of total market share in 2025. This established product category encompasses solutions featuring advanced chemical processing technologies and specialized pharmaceutical applications, including high-performance disintegration properties and enhanced tablet characteristics that enable superior pharmaceutical benefits and formulation outcomes across all manufacturing applications. The synthetic disintegrants segment's market leadership stems from its proven performance capabilities, with solutions capable of addressing diverse pharmaceutical requirements while maintaining consistent quality standards and disintegration effectiveness across all production environments.
The natural disintegrants segment maintains a substantial 40.0% market share, serving applications that require organic-based formulation systems with enhanced biocompatibility properties for pharmaceutical manufacturing and nutraceutical production. These solutions offer advanced natural processing capabilities for complex formulation applications while providing sufficient performance characteristics to meet pharmaceutical and nutraceutical operational demands.
Key product type advantages driving the synthetic disintegrants segment include:
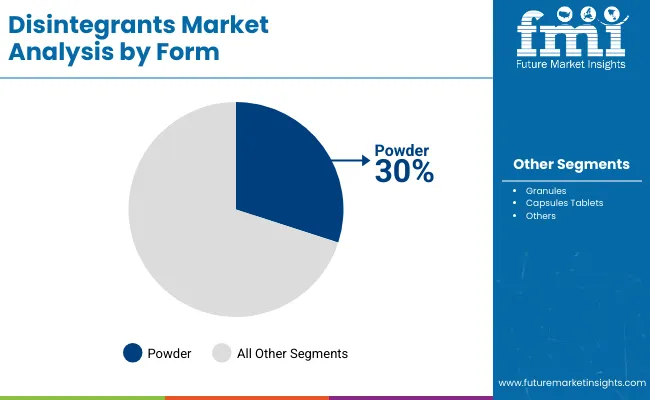
Tablet applications represent a significant category in the disintegrants market with approximately 30.0% market share in 2025, reflecting the critical role of tablet formulations in supporting specialized pharmaceutical requirements and drug delivery performance worldwide. The tablets segment's strong market position is reinforced by increasing pharmaceutical manufacturing trends, formulation complexity requirements, and rising needs for specialized disintegration capabilities in tablet applications across developed and emerging markets.
The powder segment captures 30.0% market share through specialized requirements for pharmaceutical processing, bulk formulation systems, and manufacturing applications. The granules segment represents 25.0% market share, serving intermediate processing applications that require specific particle size properties with enhanced flowability characteristics for large-scale pharmaceutical production and nutraceutical manufacturing. The capsules segment accounts for 15.0% market share, serving encapsulation applications requiring specific disintegration properties or specialized delivery configurations.
Key market dynamics supporting form growth include:
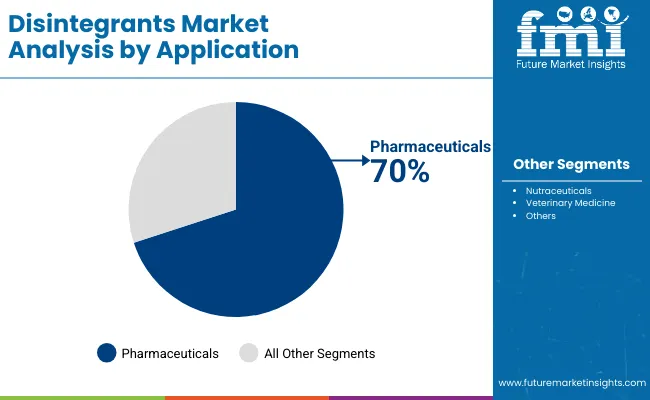
Pharmaceutical applications dominate the disintegrants market with approximately 70.0% market share in 2025, reflecting the critical role of disintegrants in supporting specialized drug formulation requirements and therapeutic performance worldwide. The pharmaceuticals segment's market leadership is reinforced by increasing drug development trends, formulation complexity requirements, and rising needs for specialized disintegration capabilities in pharmaceutical applications across developed and emerging markets.
The nutraceuticals segment represents the second-largest application category, capturing 20.0% market share through specialized requirements for dietary supplement formulations, nutritional product development, and consumer health applications. This segment benefits from growing health and wellness demand that requires specific formulation requirements, quality compliance standards, and performance optimization protocols in consumer markets.
The veterinary medicine segment accounts for 10.0% market share, serving animal health formulations, veterinary pharmaceutical products, and specialized animal care applications across various sectors.
Key market dynamics supporting application growth include:
The market is driven by three concrete demand factors tied to pharmaceutical and nutraceutical outcomes. First, advanced pharmaceutical development and specialized drug formulation solutions create increasing demand for high-performance disintegrant systems, with performance enhancement of 15-25% annually in major pharmaceutical applications worldwide, requiring comprehensive formulation infrastructure.
Second, government initiatives promoting advanced pharmaceutical technologies and drug development drive increased adoption of specialized disintegrant compounds, with many countries implementing pharmaceutical development programs and regulatory frameworks for drug advancement by 2030. Third, technological advancements in synthetic processing and pharmaceutical applications enable more efficient and effective formulation solutions that improve therapeutic performance while reducing operational costs and processing complexity.
Market restraints include complex formulation requirements and validation costs for specialized disintegrant platforms that can challenge market participants in developing compliant processing capabilities, particularly in regions where regulatory pathways for advanced pharmaceutical technologies remain evolving and uncertain.
Technical complexity of specialized disintegrant systems and quality requirements pose another significant challenge, as pharmaceutical disintegrants demand sophisticated processing methods and regulatory compliance, potentially affecting production costs and operational efficiency. Supply variability constraints from raw material availability across different regions create additional operational challenges for manufacturers, demanding ongoing investment in supply chain development and sourcing assurance programs.
Key trends indicate accelerated adoption in Asia-Pacific markets, particularly India and China, where pharmaceutical expansion and manufacturing modernization drive comprehensive disintegrant adoption. Technology integration trends toward specialized pharmaceutical systems with enhanced performance characteristics, advanced nutraceutical applications, and integrated processing solutions enable effective manufacturing approaches that optimize production efficiency and minimize formulation risks. However, the market thesis could face disruption if significant advances in alternative excipient technologies or major changes in pharmaceutical formulation practices reduce reliance on traditional disintegrant applications.
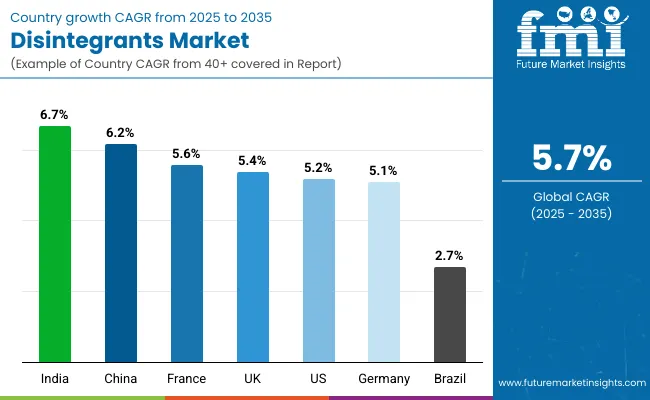
| Countries | CAGR (%) |
|---|---|
| India | 6.7 |
| China | 6.2 |
| France | 5.6 |
| UK | 5.4 |
| USA | 5.2 |
| Germany | 5.1 |
| Brazil | 2.7 |
The global disintegrants market is expanding steadily, with India leading at a 6.7% CAGR through 2035, driven by pharmaceutical growth, government industrial initiatives, and advanced manufacturing platforms. China follows at 6.2%, supported by pharmaceutical modernization, large-scale manufacturing programs, and drug development initiatives. France records 5.6%, reflecting an established landscape with growing integration in pharmaceutical and nutraceutical applications.
UK grows at 5.4%, anchored by pharmaceutical excellence and strong drug development pipelines. USA advances at 5.2%, leveraging advanced pharmaceutical manufacturing and precision formulation systems. Germany posts 5.1%, focusing on pharmaceutical integration, while Brazil grows steadily at 2.7%, emphasizing manufacturing expansion and pharmaceutical development.
India demonstrates the strongest growth potential in the disintegrants market with a CAGR of 6.7% through 2035. The country's leadership position stems from pharmaceutical sector expansion, government-backed industrial initiatives, and comprehensive drug manufacturing regulations driving the adoption of advanced disintegrant solutions.
Growth is concentrated in major pharmaceutical and manufacturing centers, including Mumbai, Hyderabad, Bangalore, and Ahmedabad, where pharmaceutical companies and generic drug manufacturers are implementing advanced disintegrant systems for enhanced production capabilities and formulation effectiveness. Distribution channels through pharmaceutical suppliers and excipient providers expand deployment across manufacturing projects and drug development initiatives. The country's Ministry of Chemicals and Fertilizers provides policy support for pharmaceutical technology modernization, including comprehensive manufacturing capability development programs.
India's position as a global generic drug manufacturing hub creates substantial demand for cost-effective, high-quality disintegrant solutions that meet international regulatory standards while maintaining competitive pricing structures. The country's pharmaceutical industry benefits from established supply chains, skilled technical workforce, and supportive regulatory environment that encourages innovation in excipient technologies. Major pharmaceutical manufacturing clusters in states like Gujarat, Maharashtra, and Telangana showcase advanced deployment of disintegrant technologies where formulation systems integrate seamlessly with existing manufacturing infrastructure and comprehensive quality management programs.
Key market factors:
In major pharmaceutical and manufacturing centers including Beijing, Shanghai, Guangzhou, and Tianjin, the adoption of comprehensive disintegrant solutions is accelerating across production projects and pharmaceutical development initiatives, driven by manufacturing scaling and government industrial programs. The market demonstrates strong growth momentum with a CAGR of 6.2% through 2035, linked to comprehensive pharmaceutical modernization and increasing focus on drug manufacturing efficiency solutions.
Chinese companies are implementing advanced disintegrant systems and processing platforms to enhance production performance while meeting growing demand in expanding pharmaceutical and nutraceutical manufacturing sectors. The country's pharmaceutical development initiatives create continued demand for disintegrant compounds, while increasing emphasis on innovation drives adoption of advanced formulation systems.
China's pharmaceutical industry transformation toward higher-value drug manufacturing and research-based development creates opportunities for sophisticated disintegrant technologies that support complex formulation requirements. The integration of traditional Chinese medicine with modern pharmaceutical manufacturing techniques generates unique demand for natural disintegrant systems that maintain cultural preferences while meeting international quality standards. Strategic partnerships between international disintegrant providers and domestic pharmaceutical companies facilitate technology transfer and local production capabilities.
Key development areas:
France's market expansion is driven by diverse pharmaceutical demand, including drug formulation development in major pharmaceutical centers and comprehensive research projects across multiple regions. The country demonstrates strong growth potential with a CAGR of 5.6% through 2035, supported by government pharmaceutical programs and industry-level drug development initiatives.
French companies face implementation challenges related to formulation complexity and scaling requirements, requiring strategic development approaches and support from specialized disintegrant partners. However, growing pharmaceutical demands and advanced manufacturing requirements create compelling business cases for disintegrant adoption, particularly in pharmaceutical areas where advanced excipients have a direct impact on therapeutic success and competitive positioning.
The French pharmaceutical industry's emphasis on innovation and quality creates demand for premium disintegrant grades that support advanced drug delivery systems and specialized formulations. Major pharmaceutical companies located in regions like Île-de-France, Rhône-Alpes, and Normandy drive demand for sophisticated disintegrant technologies that enable complex formulation development and regulatory compliance across global markets.
Market characteristics:
The UK market leads in advanced pharmaceutical innovation based on integration with drug development systems and precision formulation technologies for enhanced therapeutic performance. The country shows strong potential with a CAGR of 5.4% through 2035, driven by the modernization of existing pharmaceutical infrastructure and the expansion of advanced manufacturing facilities in major pharmaceutical areas, including London, Cambridge, Manchester, and Edinburgh.
British companies are adopting intelligent disintegrant systems for quality improvement and formulation enhancement, particularly in regions with advanced pharmaceutical requirements and drug development applications demanding comprehensive technology upgrades. Technology deployment channels through established pharmaceutical institutions and manufacturing operators expand coverage across production facilities and innovation-focused applications.
The UK's strong pharmaceutical research base and established biotechnology sector create demand for specialized disintegrant solutions that support novel drug delivery systems and innovative formulation approaches. Brexit-related regulatory changes have prompted increased focus on domestic pharmaceutical manufacturing capabilities, driving investment in advanced formulation technologies and excipient supply chain development.
Leading market segments:
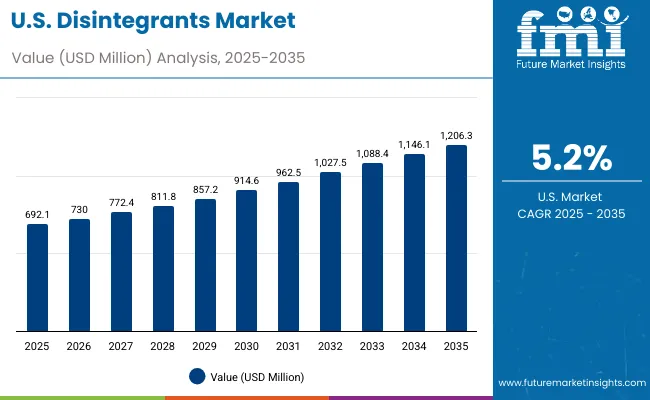
USA's disintegrants market demonstrates established and innovation-focused landscape, characterized by growing integration of formulation technologies with existing pharmaceutical infrastructure across manufacturing projects, research networks, and modernization initiatives. The country's emphasis on pharmaceutical excellence and drug development innovation drives demand for advanced disintegrant solutions that support comprehensive pharmaceutical initiatives and manufacturing requirements in production operations.
The market benefits from partnerships between international disintegrant providers and domestic pharmaceutical leaders, creating service ecosystems that prioritize formulation excellence and quality programs. Pharmaceutical centers in major regions showcase developing disintegrant implementations where formulation systems achieve efficiency improvements through integrated pharmaceutical programs.
The USA pharmaceutical market's regulatory complexity and quality requirements create demand for well-characterized, extensively tested disintegrant grades that meet FDA standards and support robust drug development programs. The presence of major pharmaceutical companies and contract manufacturing organizations drives innovation in disintegrant technologies and formulation approaches.
Germany's disintegrants market demonstrates advanced implementation focused on manufacturing precision and pharmaceutical performance optimization, with documented integration of specialized formulation systems, achieving 40% improvement in processing efficiency across pharmaceutical and nutraceutical facilities. The country maintains steady growth momentum with a CAGR of 5.1% through 2035, driven by pharmaceutical facilities' emphasis on quality excellence and continuous operational methodologies that align with German manufacturing standards applied to disintegrant operations. Major pharmaceutical areas, including Bavaria, Baden-Württemberg, North Rhine-Westphalia, and Hesse, showcase advanced deployment of disintegrant platforms where formulation systems integrate seamlessly with existing manufacturing infrastructure and comprehensive quality management programs.
Key market characteristics:
Brazil's disintegrants market demonstrates emerging growth patterns focused on pharmaceutical manufacturing expansion and local production development, with increasing adoption of disintegrant technologies in major manufacturing centers including São Paulo, Rio de Janeiro, and Minas Gerais. The country shows moderate growth potential with a CAGR of 2.7% through 2035, driven by pharmaceutical industry development and increasing focus on domestic drug manufacturing capabilities. Brazilian pharmaceutical companies are implementing disintegrant systems to enhance local production capabilities while reducing import dependencies and supporting growing domestic pharmaceutical demand.
Brazil's pharmaceutical sector benefits from established manufacturing infrastructure and favorable government policies that encourage local drug production, particularly for generic pharmaceuticals serving both domestic and regional export markets. The country's National Health Surveillance Agency provides regulatory framework support that aligns with international pharmaceutical standards while promoting domestic manufacturing capabilities. Major pharmaceutical manufacturing clusters in states like São Paulo, Rio de Janeiro, and Paraná showcase developing disintegrant implementations where formulation systems support both immediate-release and specialized tablet applications for diverse therapeutic categories.
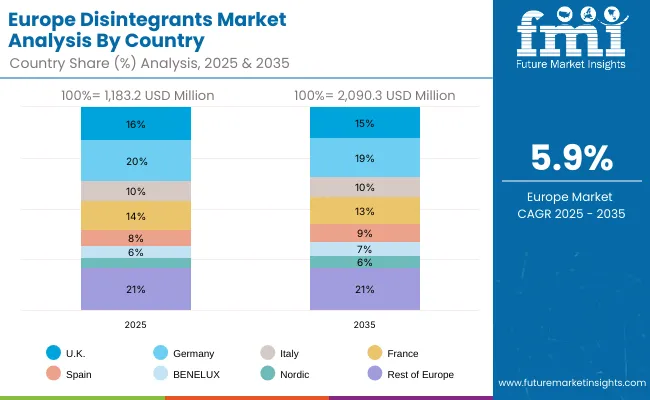
The disintegrants market in Europe is projected to grow from USD 1,183.2 million in 2025 to USD 2,090.3 million by 2035, registering a CAGR of 5.9% over the forecast period. Germany is expected to maintain its leadership position with a 20.0% market share in 2025, projected to reach 19.0% by 2035, supported by its extensive pharmaceutical infrastructure, advanced manufacturing facilities, and comprehensive research networks serving major European markets.
United Kingdom follows with a 16.0% share in 2025, projected to reach 15.0% by 2035, driven by comprehensive pharmaceutical programs in major research regions implementing advanced disintegrant systems. France holds a 14.0% share in 2025, expected to maintain 13.0% by 2035 through the ongoing development of pharmaceutical facilities and manufacturing networks. Italy commands a 10.0% share, while Spain accounts for 8.0% in 2025, projected to reach 9.0% by 2035. The Rest of Europe region is anticipated to maintain momentum, expanding its collective share from 21.0% to 21.0% by 2035, attributed to increasing disintegrant adoption in Nordic countries and emerging Eastern European pharmaceutical facilities implementing manufacturing programs.
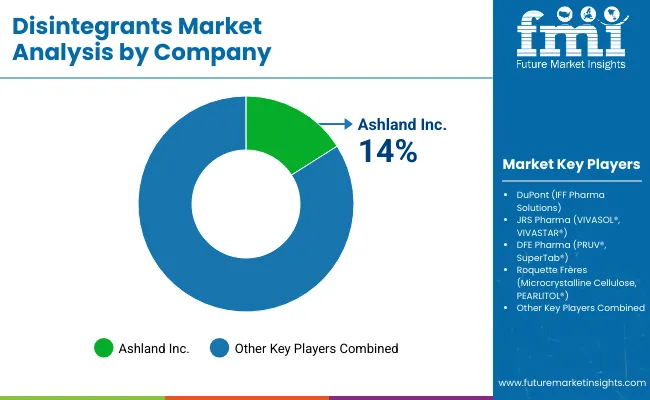
The disintegrants market features approximately 15-20 meaningful players with moderate concentration, where the top five companies control roughly 35-45% of global market share through established product portfolios and extensive pharmaceutical relationships. Competition centers on formulation capability, quality consistency, and technical expertise rather than price competition alone.
Market leaders include Ashland Inc., which holds approximately 14% market share through its established Polyplasdone and Benecel product lines, followed by DuPont with its IFF Pharma Solutions division, and JRS Pharma with its VIVASOL and VIVASTAR product portfolios. These companies maintain competitive advantages through comprehensive disintegrant portfolios, advanced processing capabilities, and deep expertise in the pharmaceutical and nutraceutical sectors, creating high switching costs for customers.
DFE Pharma competes with its PRUV and SuperTab product lines, while Roquette Frères leverages its microcrystalline cellulose and PEARLITOL technologies to serve pharmaceutical and nutraceutical applications. These companies leverage established pharmaceutical relationships and ongoing development partnerships to defend market positions while expanding into adjacent pharmaceutical and nutraceutical applications.
Challengers encompass DFE Pharma and Roquette Frères, which compete through specialized processing technologies and strong regional presence in key pharmaceutical markets. Excipient specialists focus on specific disintegrant types or vertical applications, offering differentiated capabilities in synthetic systems, natural applications, and application-specific processing.
Regional players and emerging disintegrant companies create competitive pressure through innovative processing approaches and rapid development capabilities, particularly in high-growth markets including India and China, where local presence provides advantages in cost optimization and regulatory compliance. Market dynamics favor companies that combine advanced processing technologies with comprehensive pharmaceutical services that address the complete formulation lifecycle from development through ongoing performance assurance and technical support.
Disintegrant solutions represent a critical pharmaceutical excipient that enables pharmaceutical companies, nutraceutical manufacturers, and contract manufacturing organizations to enhance formulation efficiency and product quality without substantial ongoing excipient investment, typically providing 40-60% performance enhancement compared to conventional alternatives while ensuring unprecedented reliability and regulatory compliance.
With the market projected to grow from USD 3,656.7 million in 2025 to USD 6,366.7 million by 2035 at a 5.7% CAGR, these solutions offer compelling advantages - superior performance, enhanced effectiveness, and formulation capabilities - making them essential for pharmaceutical applications (70.0% market share), nutraceutical operations (20.0% share), and diverse manufacturing applications seeking reliable disintegrant solutions. Scaling market penetration and formulation capabilities requires coordinated action across pharmaceutical policy, manufacturing standards, disintegrant providers, pharmaceutical companies, and regulatory institutions.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 3,656.7 Million |
| Product Type | Natural Disintegrants, Synthetic Disintegrants |
| Form | Powder, Granules, Capsules, Tablets |
| Application | Pharmaceuticals, Nutraceuticals, Veterinary Medicine |
| Source | Plant-Based, Microbial-Based, Mineral-Based, Synthetic |
| Sales Channel | B2B (API Manufacturers, Formulators, CDMOs), B2C (Retail Pharmacies) |
| Functionality | Superdisintegrants , Conventional Disintegrants |
| End User | Pharmaceutical Companies, Contract Manufacturing Organizations, Nutraceutical Companies |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Countries Covered | India, China, USA, Germany, France, UK, Brazil, and 40+ countries |
| Key Companies Profiled | Ashland Inc., DuPont, JRS Pharma, DFE Pharma, Roquette Frères |
| Additional Attributes | Dollar sales by product type and application categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with disintegrant providers and pharmaceutical companies, manufacturing facility requirements and specifications, integration with pharmaceutical initiatives and nutraceutical platforms. |
The global disintegrants market is estimated to be valued at USD 3,656.7 million in 2025.
The market size for the disintegrants market is projected to reach USD 6,366.7 million by 2035.
The disintegrants market is expected to grow at a 5.7% CAGR between 2025 and 2035.
The key product types in the disintegrants market are natural disintegrants and synthetic disintegrants.
In terms of product type, the synthetic disintegrants segment is expected to command 60.0% share in the disintegrants market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA