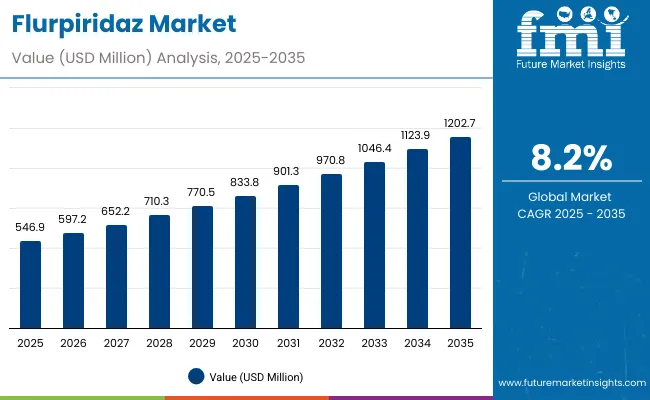
The Flurpiridaz Market has been forecasted to attain a valuation of USD 546.9 million in 2025, rising to USD 1,202.6 million by 2035, resulting in an incremental gain of USD 655.8 million, which reflects a 45.5% increase over the forecast decade. A compound annual growth rate (CAGR) of 12.1% is expected to be recorded, indicating a rapid expansion trajectory for the flurpiridaz market.
Flurpiridaz Market Key Takeaways
| Metric | Value |
|---|---|
| Flurpiridaz Market Estimated Value in (2025E) | USD 546.9 million |
| Flurpiridaz Market Forecast Value in (2035F) | USD 1,202.6 million |
| Forecast CAGR (2025 to 2035) | 8.2% |
Between 2025 and 2030, Flurpiridaz will gain broader adoption across advanced cardiology centers, hospitals, and PET imaging facilities, as clinicians increasingly rely on high-resolution PET myocardial perfusion imaging to improve diagnostic accuracy in patients with suspected coronary artery disease. It will grow up to USD 264.1 million in that period from USD 546.9 million in 2025 to USD 811.0 million in 2030. The convergence of regulatory approvals, expanding reimbursement coverage, and the rising installed base of PET/CT scanners is making Flurpiridaz more accessible, prompting uptake even in mid-tier hospitals and regional imaging networks.
Between 2030 and 2035, the market is projected to expand further by USD 391.7million, driven by advances in automated radiopharmaceutical production, broader distribution infrastructure, and integration of AI-powered cardiac image analysis. Demand will rise from both developed healthcare systems and emerging markets, as cardiologists and nuclear medicine specialists adopt Flurpiridaz for its superior image quality, lower radiation dose, and improved prognostic value compared with conventional SPECT tracers. Continuous progress in cyclotron technologies, logistics networks, and hybrid PET/MR imaging will shape the next generation of cardiac PET diagnostics.
From 2020 to 2024, the flurpiridaz market, expanded from USD 353.8 million to USD 508.6 million, as pivotal trial data validated its clinical superiority and manufacturers invested in production capabilities. During this phase, global radiopharmaceutical companies partnered with academic medical centers to streamline synthesis processes, ensure dose stability, and establish efficient supply chain pathways laying the foundation for large-scale commercial rollout.
Going forward, the Flurpiridaz ecosystem will likely evolve to incorporate real-time quantitative perfusion analysis, AI-enhanced diagnostic workflows, and hybrid compatibility with PET/MR systems. These innovations aim to reduce variability in cardiac imaging, expand usability across diverse healthcare environments, and improve patient throughput making Flurpiridaz a cornerstone tracer in the global nuclear cardiology landscape.
The growth of the Flurpiridaz Market is reinforced by the unique clinical value of this PET myocardial perfusion imaging (MPI) tracer in diagnosing coronary artery disease (CAD) the world’s leading cause of mortality. In contrast to conventional SPECT tracers, Flurpiridaz provides higher image resolution, improved sensitivity for detecting perfusion defects, and more accurate quantification of blood flow, making it indispensable for modern cardiovascular imaging.
Over the last decade, the scope of Flurpiridaz has expanded beyond early-phase trials into broad clinical readiness. It is now positioned as a critical diagnostic tool in cardiology, enabling earlier and more precise detection of ischemia, particularly in patients with multi-vessel disease or obesity, where SPECT accuracy declines. As cardiovascular disease prevalence rises globally and health systems seek more reliable, lower-radiation diagnostic solutions, Flurpiridaz is emerging as a next-generation standard for myocardial perfusion imaging.
Another factor driving adoption is the transition from limited research-center availability to scalable, commercial production. With radiopharmaceutical companies investing in distribution infrastructure, automated synthesis modules, and cyclotron capacity, Flurpiridaz can now be deployed widely across PET/CT facilities. This decentralization is transforming its accessibility, particularly in healthcare systems expanding nuclear cardiology capabilities.
Moreover, increasing demand from complementary fields including personalized medicine, quantitative perfusion analysis, and hybrid PET/MR imaging is fostering the integration of Flurpiridaz into AI-powered diagnostic workflows. These advances cater to the growing need for precision, efficiency, and reproducibility in cardiovascular care.
Collectively, these clinical and technological drivers are positioning the Flurpiridaz market for sustained expansion, fueled by its essential role in improving cardiac diagnostics and enabling better patient outcomes in the era of precision medicine.
The market is segmented by technology, application, end user, and region. Technology includes PET MPI (positron emission tomography myocardial perfusion imaging), PET/CT systems, and PET/MR systems. Application covers coronary artery disease (CAD) diagnosis, myocardial ischemia and infarction assessment, and advanced cardiac diagnostics. Based on end user, the segmentation includes hospitals and medical centers, diagnostic imaging centers, independent PET imaging centers, outpatient diagnostic centers, research institutions and academic centers, and government and defense medical facilities. Regionally, the scope spans North America, Latin America, Western and Eastern Europe, East Asia, South Asia and Pacific, and the Middle East and Africa.
| Technology | Market Value Share, 2025 |
|---|---|
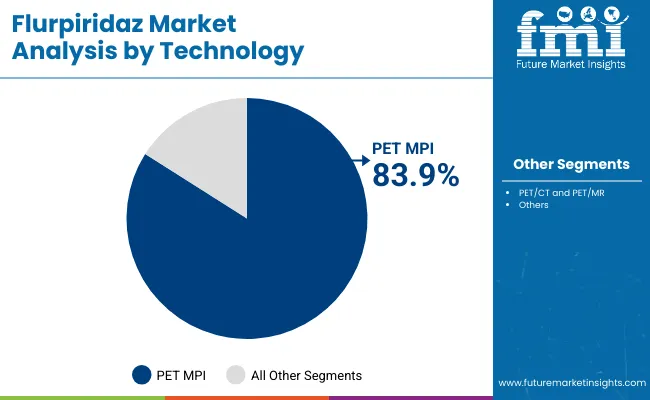
| PET MPI (Positron Emission Tomography Myocardial Perfusion Imaging) | 83.9% |
| PET/CT and PET/MR | 16.1% |
PET MPI (positron emission tomography myocardial perfusion imaging) is projected to lead the technology category with an 83.9% market share in 2025, and is expected to maintain this position due to its ability to provide high-resolution, quantitative assessment of myocardial blood flow. Unlike conventional SPECT imaging, PET MPI offers superior spatial resolution, higher sensitivity, and more accurate perfusion quantification allowing cardiologists to detect subtle ischemic changes and multi-vessel disease with unprecedented precision.
Recent advances in radiotracer production, including automated synthesis modules for flurpiridaz, combined with optimized PET scanner calibration and image reconstruction algorithms, have enabled these systems to deliver consistent, reproducible results across clinical settings. Moreover, integration with advanced software for AI-assisted perfusion analysis allows clinicians to assess myocardial function, ischemia, and infarction risk in real time making PET MPI indispensable for modern cardiac diagnostics.
A key factor behind their dominance is their widespread adoption in hospitals, diagnostic imaging centers, and specialized PET facilities. With commercial vendors now offering turnkey systems that simplify workflow and tracer handling, PET MPI is becoming increasingly accessible to mid-tier hospitals and regional cardiology centers. Its relatively lower radiation dose and faster acquisition times further position it as the preferred imaging modality for precision cardiology.
As cardiovascular disease prevalence continues to rise and personalized medicine gains prominence, the diagnostic accuracy, workflow efficiency, and quantitative capabilities of PET MPI ensure that it remains the backbone of cardiac perfusion imaging across clinical and research settings alike.
| Application | Market Value Share, 2025 |
|---|---|
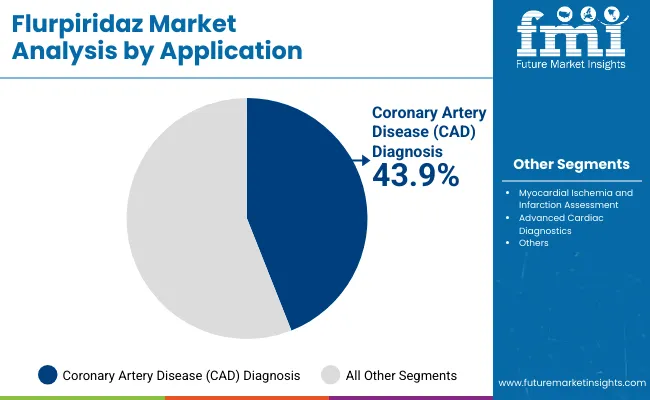
| Coronary Artery Disease (CAD) Diagnosis | 43.9% |
| Myocardial Ischemia and Infarction Assessment | 37.0% |
| Advanced Cardiac Diagnostics | 19.1% |
Coronary artery disease (CAD) diagnosis continues to dominate the application landscape, accounting for 43.9% of total demand in 2025. This application remains the foundation of cardiac imaging due to Flurpiridaz PET MPI’s proven ability to detect perfusion defects, quantify myocardial blood flow, and assess ischemic burden with high sensitivity and spatial resolution. As an essential tool in modern cardiology, Flurpiridaz provides unmatched insight into patient-specific coronary physiology, enabling clinicians to make informed treatment decisions.
What makes Flurpiridaz indispensable in CAD diagnosis is its reproducibility, quantitative accuracy, and established clinical validation? Many hospitals and imaging centers have developed expertise in PET MPI workflows, and longitudinal studies using Flurpiridaz have created extensive datasets that support comparative assessments, risk stratification, and prognostic evaluation. The tracer is particularly effective in patients with multi-vessel disease, obesity, or prior revascularization, where conventional SPECT imaging may be less reliable.
Recent improvements in tracer distribution networks, PET scanner calibration, and image reconstruction algorithms have further enhanced the resolution, usability, and clinical throughput of Flurpiridaz for CAD assessment. Moreover, cardiology training programs and nuclear medicine curricula continue to emphasize PET MPI with Flurpiridaz as a primary diagnostic modality, ensuring a steady pipeline of skilled operators and adoption in clinical practice.
While applications such as myocardial ischemia and infarction assessment and advanced cardiac diagnostics are growing, CAD diagnosis remains the mainstay of the global Flurpiridaz market, largely due to its clinical impact, diagnostic accuracy, and broad compatibility across hospitals, imaging centers, and research institutions.
The adoption of Flurpiridaz in cardiac imaging is accelerating as clinical and technological advancements converge to improve the detection and assessment of myocardial perfusion. While demand is growing, the market faces structural challenges due to regulatory complexities, high development costs, and the need for specialized imaging infrastructure.
Rising prevalence of cardiovascular diseases
Cardiovascular diseases (CVDs) remain the leading cause of mortality, with coronary artery disease (CAD) representing a significant portion of the global burden. The increasing incidence of CAD, myocardial ischemia, and related complications has created an urgent need for early, accurate, and non-invasive diagnostic solutions. Flurpiridaz, as a PET myocardial perfusion imaging (MPI) tracer, offers superior spatial resolution and quantitative perfusion assessment compared with conventional SPECT tracers, enabling precise evaluation of blood flow, ischemia, and infarction risk. Clinicians rely on these capabilities for early detection, risk stratification, and treatment planning, particularly in high-risk patients, multi-vessel disease cases, and populations where SPECT imaging is less reliable, such as obese patients. Moreover, the global shift toward personalized medicine is further driving the adoption of advanced imaging modalities that provide patient-specific insights. Hospitals, diagnostic imaging centers, and research institutions increasingly prefer Flurpiridaz due to its reproducibility, validated efficacy, and ability to improve clinical decision-making. As the prevalence of CVD continues to rise exacerbated by aging populations, sedentary lifestyles, and comorbidities such as diabetes and hypertension the demand for Flurpiridaz is expected to expand steadily, positioning it as a key tool in modern cardiac diagnostics.
Integration of AI-powered image analysis with PET imaging
AI-powered image analysis enhances the accuracy, reproducibility, and efficiency of cardiac diagnostics by automating the quantification of myocardial perfusion, ventricular function, and ischemic burden. This reduces inter-observer variability and allows clinicians to detect subtle perfusion defects that may be overlooked using conventional methods. Additionally, AI integration enables real-time image reconstruction and predictive analytics, supporting faster diagnosis and better patient management. Coupled with hybrid PET/CT and PET/MR systems, AI tools can combine anatomical and functional information, providing a comprehensive assessment of cardiac health in a single session. Research institutions and advanced hospitals are increasingly leveraging these capabilities for both clinical applications and cardiac research, driving adoption of Flurpiridaz as part of next-generation diagnostic workflows. Furthermore, the trend toward remote and cloud-based AI platforms allows smaller facilities to access advanced image analysis without significant infrastructure investments, potentially expanding the tracer’s reach.
High costs and limited PET infrastructure
PET scanners and PET/CT or PET/MR hybrid systems require substantial capital investment, making them inaccessible to smaller hospitals, regional imaging centers, and facilities in emerging markets. In addition, production of Flurpiridaz relies on cyclotron-generated radiotracers, which necessitates specialized infrastructure, maintenance, and highly trained personnel for synthesis, quality control, and safe handling. The need for robust distribution networks, cold-chain storage, and rapid delivery further complicates widespread deployment. These factors collectively increase the overall cost of the procedure for healthcare providers and patients, limiting adoption despite the tracer’s clinical benefits. Moreover, in regions with underdeveloped nuclear medicine infrastructure, the lack of trained nuclear medicine physicians, technologists, and radiopharmacists restricts effective utilization. Regulatory requirements and licensing for radioactive materials can also slow market penetration. As a result, while demand is growing in well-equipped urban centers and advanced healthcare systems, high infrastructure costs and limited access in smaller hospitals or rural areas remain significant restraints for the Flurpiridaz market.
| Country | CAGR |
|---|---|
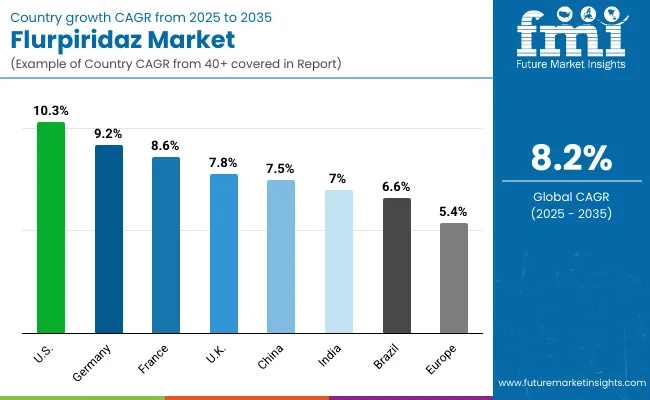
| USA | 10.3% |
| Brazil | 6.6% |
| China | 7.5% |
| India | 7.0% |
| Europe | 6.9% |
| Germany | 9.2% |
| France | 8.6% |
| UK | 7.8% |
The adoption and growth patterns of Flurpiridaz in the United States exhibit distinct variations across different healthcare settings, influenced by the maturity of medical infrastructure, regulatory support, and demand for advanced cardiac imaging.
In the United States, the Flurpiridaz market is characterized by strong growth driven by a robust network of nuclear cardiology centers, leading academic hospitals, and ongoing government- and industry-funded clinical research. The availability of state-of-the-art PET imaging systems and increasing focus on non-invasive, high-accuracy myocardial perfusion imaging supports widespread adoption. The presence of specialized cardiac research programs and continuous investments in cardiovascular diagnostics propels demand for Flurpiridaz, particularly in areas like early detection of coronary artery disease and precise assessment of myocardial perfusion. The market here is projected to grow at a CAGR of 10.3%.
In Europe, countries such as Germany, France, and the UK lead the Flurpiridaz market with growing emphasis on integrating advanced cardiac imaging agents within national healthcare initiatives and cardiovascular research programs. Government-backed clinical studies, reimbursement support, and investment in state-of-the-art PET imaging infrastructure have facilitated collaboration among hospitals, academic institutions, and diagnostic centers, enabling the adoption of Flurpiridaz for precise myocardial perfusion assessment. Regulatory frameworks that support innovative imaging solutions and strategic funding for cardiovascular care foster sustained market growth, though adoption remains measured as clinical familiarity and long-term outcomes continue to accumulate. The market in Germany is expected to expand at a CAGR of 9.2%, France at 8.6%, and the UK at 7.8%.
Overall, the European Flurpiridaz market reflects a strategic combination of clinical need, healthcare policy alignment, and institutional investment, which together govern the pace and scale of adoption across countries. This dynamic interplay underscores the central role that regional regulatory frameworks, imaging infrastructure, and clinical expertise play in shaping the trajectory of this next-generation cardiac imaging agent.
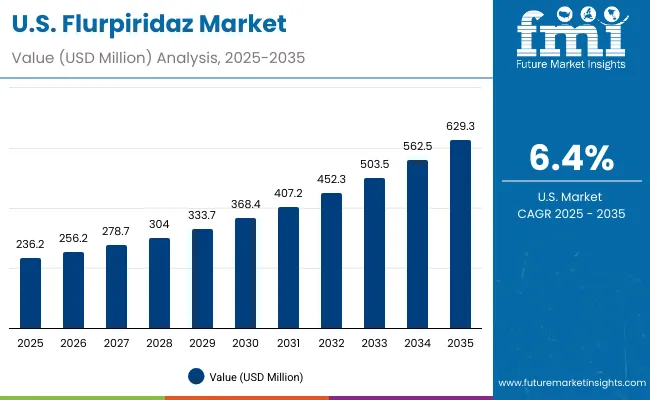
The Flurpiridaz market in the United States has been forecasted to expand at a CAGR of 10.3% between 2025 and 2035. The USA remains the most mature and research-intensive market for cardiac PET imaging agents, with widespread integration into nuclear cardiology programs and advanced cardiac diagnostic centers. Cleveland Clinic, Mayo Clinic, and Johns Hopkins Hospital, alongside government-supported research initiatives, have been pivotal in establishing clinical protocols and validating Flurpiridaz for myocardial perfusion imaging. The availability of state-of-the-art PET/CT systems and advanced radiopharmacy facilities ensures reliable access to Flurpiridaz across diagnostic networks.
India's Flurpiridaz market is projected to grow at a CAGR of 7.0% from 2025 to 2035, making it one of the fastest-growing national markets for advanced cardiac imaging globally. This growth is driven by rising cardiovascular disease prevalence, expanding PET imaging infrastructure, and adoption of advanced nuclear cardiology workflows. India’s market growth is influenced by its dependency on imported radiopharmaceutical components, which directly links to global supply chains and availability of Flurpiridaz. Flurpiridaz is an F18 PET tracer, its production in India requires cyclotrons, hot cells, chemistry modules, and enriched target materials, most of which are imported from multinational suppliers. This creates a connection between India’s domestic adoption rate and the global production and distribution of Flurpiridaz.
China’s Flurpiridaz market is projected to grow at a CAGR of 7.5% between 2025 and 2035. According to a 2024 survey published in the Journal of Nuclear Medicine, China had 1,148 nuclear medicine departments, representing a more than twofold increase over the past decade. This rapid expansion reflects significant growth in nuclear cardiology infrastructure, driven by rising cardiovascular disease prevalence, government initiatives to modernize healthcare, and increased investment in PET/CT imaging systems
| Country | 2025 |
|---|---|
| Germany | 28.0% |
| UK | 23.1% |
| France | 17.2% |
| Italy | 12.1% |
| Spain | 8.3% |
| BENELUX | 6.0% |
| Nordic Countries | 3.4% |
| Rest of Western Europe | 1.8% |
| Country | 2035 |
|---|---|
| Germany | 25.6% |
| UK | 22.9% |
| France | 17.4% |
| Italy | 12.2% |
| Spain | 8.9% |
| BENELUX | 6.3% |
| Nordic Countries | 4.1% |
| Rest of Western Europe | 2.7% |
The Flurpiridaz market in the United Kingdom is projected to grow at a CAGR of 7.8% from 2025 to 2035. The country benefits from robust public investment in diagnostic infrastructure, including the 2025/26 NHS settlement, which allocated £1.65 billion to improve NHS performance, with £0.134 billion dedicated specifically to diagnostic networks. This funding supports the integration of advanced imaging modalities, including PET/CT scanners essential for myocardial perfusion studies.Flurpiridaz adoption is primarily concentrated in regions with established nuclear cardiology programs, supported by centralized distribution systems and coordinated training frameworks. Ongoing government initiatives facilitate the standardization of imaging protocols and knowledge sharing across institutions, enabling efficient implementation of PET imaging workflows.
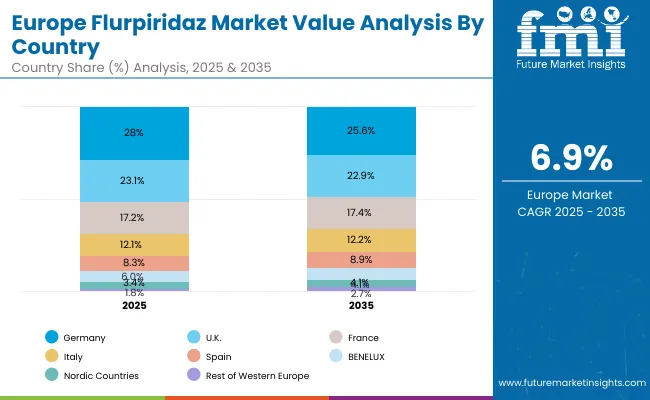
The Flurpiridaz market in Germany is projected to grow at a CAGR of 9.2% from 2025 to 2035, supported by increasing cardiovascular disease prevalence and strategic investments in nuclear cardiology infrastructure. The country’s advanced diagnostic imaging ecosystem enables widespread adoption of PET myocardial perfusion imaging, with emphasis on modernizing workflows and integrating high-resolution imaging capabilities.Growth is concentrated in regions with high diagnostic demand, including Bavaria, Baden-Württemberg, and North Rhine-Westphalia. National programs facilitate standardization of imaging protocols and the optimization of clinical workflows, while research initiatives provide access to cutting-edge imaging technologies for high-precision cardiac assessments.
| Technology | Market Value Share, 2025 |
|---|---|
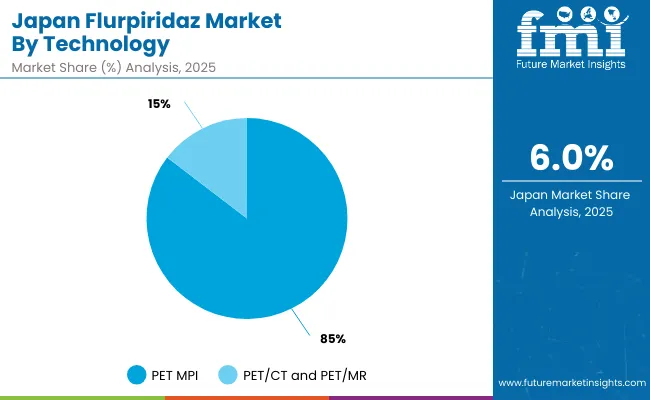
| PET MPI (Positron Emission Tomography Myocardial Perfusion Imaging) | 85.4% |
| PET/CT and PET/MR | 14.6% |
The Flurpiridaz Market in Japan has been projected to reach USD 15.86 million in 2025. Japan’s PET myocardial perfusion imaging (PET MPI) market is characterized by steady clinical demand, driven by the country’s focus on early detection of coronary artery disease, high standards of diagnostic quality, and widespread adoption of advanced imaging technologies. The market is dominated by PET MPI, due to its superior sensitivity and specificity for myocardial perfusion assessment compared to conventional SPECT imaging. While hybrid PET/CT and PET/MR systems are gradually increasing in adoption, as hospitals and diagnostic centers prioritize PET MPI for routine cardiac evaluation.The Japanese healthcare ecosystem includes highly regulated diagnostic centers and cardiology-focused institutions that maintain strict imaging protocols and standardized tracer usage. The market’s growth is supported by increasing cardiovascular disease prevalence, regulatory emphasis on high-quality diagnostic standards, and ongoing infrastructure upgrades in imaging facilities.
| Application | Market Value Share, 2025 |
|---|---|
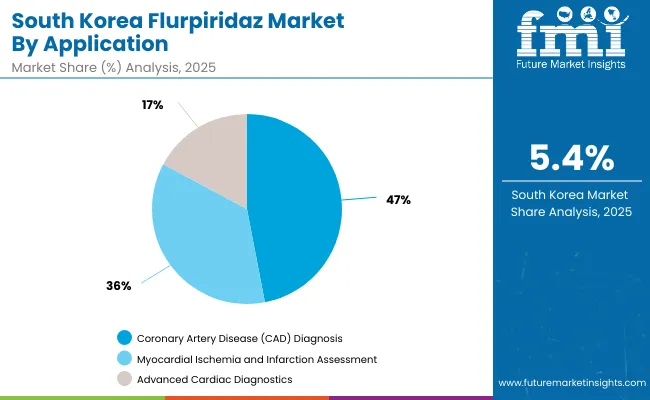
| Coronary Artery Disease (CAD) Diagnosis | 47.0% |
| Myocardial Ischemia and Infarction Assessment | 35.8% |
| Advanced Cardiac Diagnostics | 17.2% |
The Flurpiridaz Market in South Korea has been projected to reach USD 10.39 million in 2025. South Korea’s cardiac imaging market is experiencing robust growth, driven by increasing prevalence of cardiovascular diseases and ongoing investment in advanced diagnostic infrastructure. The Coronary Artery Disease (CAD) Diagnosis segment leads the market, accounting for 47.0% of adoption, as accurate early detection remains a clinical priority. The Myocardial Ischemia and Infarction Assessment segment follows with 35.8% share, reflecting widespread use of PET MPI and related imaging techniques to guide treatment planning emphasizing high-quality cardiac care, standardized imaging protocols, and integration of advanced PET and hybrid imaging modalities in clinical workflows. The market’s steady growth is supported by rising demand for early and precise cardiovascular diagnosis, increasing infrastructure investment, and adherence to national and international clinical standards.
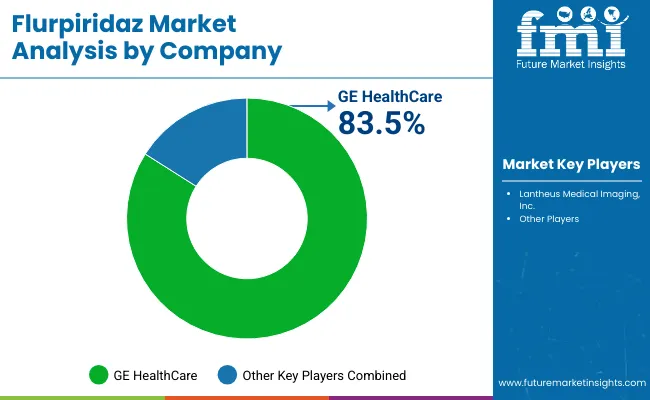
| Company | Value Share 2024 |
|---|---|
| GE HealthCare | 83.5% |
| Others | 16.5% |
The Flurpiridaz market is highly concentrated, with GE Healthcare holding a dominant global position due to its extensive portfolio of PET/CT imaging systems and integrated workflow solutions for myocardial perfusion imaging. The company’s market leadership is anchored in its ability to provide end-to-end imaging solutions, including optimized PET/CT hardware, AI-assisted analysis software, and standardized imaging protocols. GE Healthcare’s established presence in cardiovascular diagnostics and broad clinical support network further reinforces its dominant position in the market.
Other players in the market include Lantheus Medical Imaging, Inc., focused on radiotracer supply and niche cardiac imaging solutions, supporting adoption in specialized centers.
Key Developments in Flurpiridaz Market
| Item | Value |
|---|---|
| Quantitative Units | USD 546.9 million |
| By Technology | PET MPI (Positron Emission Tomography Myocardial Perfusion Imaging), PET/CT and PET/MR |
| Application Type | Coronary Artery Disease (CAD) Diagnosis, Myocardial Ischemia and Infarction Assessment, Advanced Cardiac Diagnostics |
| End User | Hospitals and Medical Centers, Diagnostic Imaging Centers, Independent PET Imaging Centers, Outpatient Diagnostic Centers, Research Institutions & Academic Centers, Government and Defense Medical Facilities |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa |
| Countries Covered | USA, Brazil, China, India, Europe, Germany, France and UK |
| Key Companies Profiled | GE HealthCare, Lantheus Medical Imaging, Inc. |
The global flurpiridaz market is estimated to be valued at USD 546.9million in 2025.
The market size for the global flurpiridaz market is projected to reach approximately USD 1,202.6 million by 2035.
The global flurpiridaz market is expected to grow at a CAGR of 8.2% between 2025 and 2035.
The key technology types in the global flurpiridaz market include PET MPI (Positron Emission Tomography Myocardial Perfusion Imaging), PET/CT and PET/MR.
In terms of application, Coronary Artery Disease (CAD) Diagnosis is projected to command the highest share at 43.9% in the global flurpiridaz market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA