The phage MuA transposase market is valued at USD 321.1 million in 2025 and is expected to reach USD 726.0 million by 2035, with a CAGR of 8.5%. The market begins its growth journey from USD 213.5 million in 2021, progressing to USD 321.1 million by 2025. Intermediate values in this period include USD 231.7 million, 251.4 million, 272.7 million, and 295.9 million.
This phase is characterized by steady growth as the technology is increasingly adopted in genetic engineering, pharmaceutical research, and biotechnological applications. The growing demand for advanced genetic tools for drug development, diagnostics, and biotechnology fuels the early-stage market accumulation.
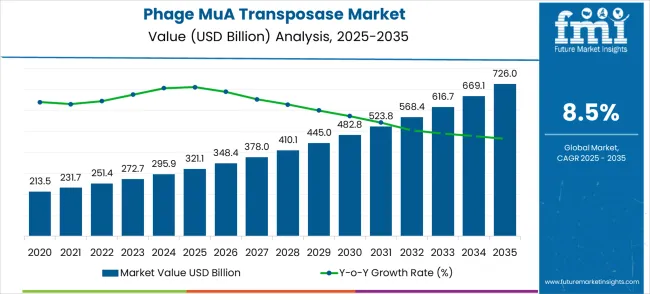
Between 2026 and 2030, the market expands significantly, from USD 321.1 million to USD 482.8 million, with intermediate values of USD 348.4 million, 378.0 million, 410.1 million, and 445.0 million. This period sees a more pronounced growth as the technology becomes more integral in various applications, including gene therapy and genome editing.
The adoption of more advanced MuA transposase-based tools in clinical and industrial settings drives the market further, with product innovations and collaborations between biotech companies contributing to rapid growth. By 2030, the market will reach USD 523.8 million, and from 2031 to 2035, it will continue its upward trajectory, reaching USD 726.0 million, driven by increasing commercialization, technological advancements, and expanding global healthcare and biotech sectors.
The biotechnology market contributes about 35-40%, as MuA transposase plays a critical role in genetic research, gene editing, and the development of gene therapies, especially in the study of DNA transposition mechanisms. The genomics and molecular biology market adds roughly 25-30%, with MuA transposase being an essential tool in genomic manipulation, such as transposon-mediated mutagenesis, cloning, and DNA insertion techniques, which are widely used in research and pharmaceutical development.
The pharmaceutical market contributes around 15-18%, driven by the growing need for advanced tools for drug discovery, gene therapy development, and precision medicine, where MuA transposase is used for the creation of gene constructs and the development of genetic modifications.
The agriculture and plant biotechnology market accounts for approximately 10-12%, as MuA transposase is utilized in genetic engineering of crops, enabling the introduction of specific traits through transposon-based systems. The diagnostics market contributes about 8-10%, where MuA transposase is used in diagnostic assays and tools for detecting and manipulating specific genes or sequences in clinical and research settings
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 321 million |
| Forecast Value in (2035F) | USD 726.0 million |
| Forecast CAGR (2025 to 2035) | 8.5% |
Market expansion is being supported by the increasing complexity of biotechnology research and the corresponding need for precise molecular biology tools that can facilitate advanced genetic engineering, gene expression studies, and therapeutic protein development applications.
Modern biotechnology research requires sophisticated enzymatic tools that can provide reliable transposition activities, consistent performance characteristics, and comprehensive experimental reproducibility across diverse research applications. Phage MuA transposase provides the necessary specificity, efficiency, and reliability to ensure optimal research outcomes in demanding molecular biology and genetic engineering applications.
The growing emphasis on personalized medicine and gene therapy development is driving demand for advanced transposase enzymes that can support precision genetic modifications, targeted therapeutic interventions, and comprehensive functional genomics studies.
Biotechnology companies are implementing sophisticated research programs that require reliable enzymatic tools for therapeutic target identification, drug mechanism studies, and biomarker discovery applications. Regulatory requirements and research quality standards are establishing comprehensive validation protocols that require specialized enzyme technologies with proven performance characteristics and extensive quality assurance documentation.
The market is segmented by enzyme type, end-use industry, and region. By enzyme type, the market is divided into standard active enzyme and high concentration enzyme Based on end-use industry, the market is categorized into biopharmaceuticals, vaccine development, functional genomics research, and others. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
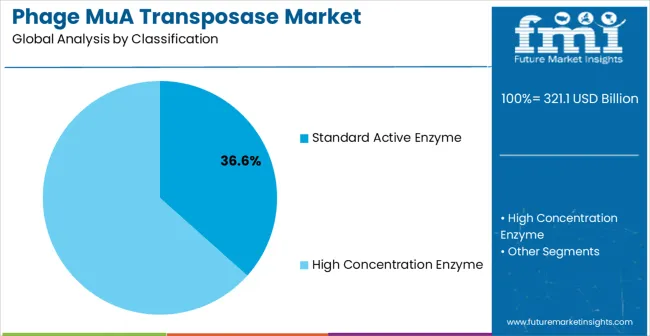
Standard active enzyme formulations are projected to account for 36.6% of the phage MuA transposase market in 2025. This leading share is supported by the balanced performance characteristics and cost-effectiveness that standard formulations provide for routine molecular biology applications and research protocols.
Standard active transposase enzymes offer reliable transposition efficiency, consistent quality performance, and proven compatibility with diverse experimental conditions that make them preferred choices for academic research institutions, biotechnology companies, and pharmaceutical research laboratories. The segment benefits from established manufacturing processes, comprehensive quality control protocols, and extensive application databases that facilitate adoption across diverse research applications.
Standard active enzyme transposase technology continues advancing through optimization of enzyme formulations, enhanced stability characteristics, and improved storage conditions that support reliable research applications and extended shelf life requirements. The segment growth reflects increasing adoption of standard formulations in expanding research institutions and growing biotechnology companies that require cost-effective yet reliable enzymatic tools for diverse molecular biology applications. Manufacturers are developing next-generation standard active enzymes with enhanced specific activity, improved temperature stability, and comprehensive quality assurance that address evolving research requirements while maintaining competitive cost structures for high-volume research applications.
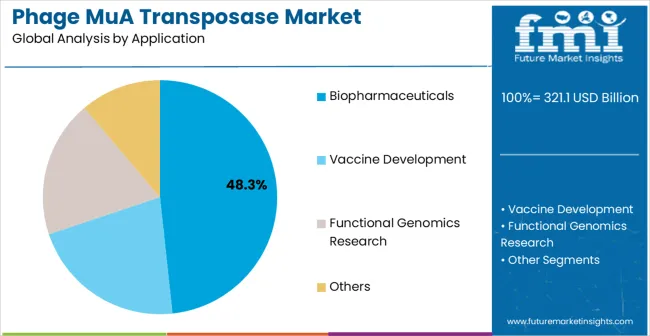
Biopharmaceuticals applications are expected to represent 48.3% of phage MuA transposase demand in 2025. This dominant share reflects the extensive use of transposase enzymes in pharmaceutical research, drug discovery programs, and therapeutic development applications that require precise genetic modifications and comprehensive functional studies.
Modern biopharmaceutical research depends on advanced molecular biology tools including transposase enzymes for target validation, mechanism of action studies, and therapeutic protein engineering that support drug development pipelines and regulatory approval processes. The segment benefits from ongoing pharmaceutical industry investments, increasing emphasis on personalized medicine approaches, and growing demand for advanced therapeutic modalities including cell and gene therapies.
Biopharmaceuticals industry transformation toward precision medicine and targeted therapeutic approaches is driving significant transposase enzyme demand as companies implement sophisticated research programs for biomarker discovery, therapeutic target identification, and drug mechanism validation.
The segment expansion reflects increasing emphasis on molecular understanding of disease mechanisms, precision therapeutic interventions, and comprehensive safety assessment that depend on reliable transposase tools and advanced genetic engineering capabilities. Advanced pharmaceutical applications are incorporating high-throughput screening approaches, automated research workflows, and comprehensive data analysis that require sophisticated enzyme technologies with validated performance characteristics and comprehensive quality documentation.
The phage MuA transposase market is advancing rapidly due to increasing biotechnology research investments and growing demand for advanced molecular biology tools. The market faces challenges including high development costs for specialized enzyme formulations, complex quality control requirements, and need for extensive validation protocols. Technological advancement efforts and research standardization programs continue to influence product development and market expansion patterns.
The growing implementation of automated research systems and high-throughput screening applications is driving demand for transposase enzymes that can provide consistent performance in robotic workflows, automated liquid handling systems, and comprehensive screening protocols. Advanced automation-compatible enzyme formulations enable efficient research operations, reduced manual intervention, and comprehensive data generation that support modern biotechnology research requirements. These technological advances enable research organizations to achieve higher levels of experimental throughput and data quality while reducing operational costs and improving research reproducibility through standardized automated approaches.
Transposase enzyme manufacturers are developing specialized formulations and application-specific solutions that address unique research requirements including temperature sensitivity, buffer compatibility, and substrate specificity optimization. Next-generation enzyme products provide enhanced performance characteristics, improved stability profiles, and comprehensive compatibility that support diverse research applications and experimental conditions. These product innovations support broader market adoption by providing tailored solutions that meet specific research needs while maintaining superior performance characteristics and comprehensive quality assurance for demanding biotechnology applications.
| Country | CAGR (2025-2035) |
|---|---|
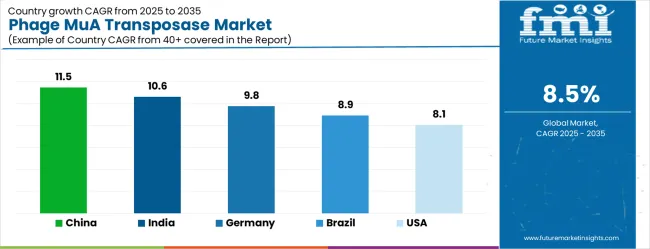
| China | 11.5% |
| India | 10.6% |
| Germany | 9.8% |
| Brazil | 8.9% |
| United States | 8.1% |
| United Kingdom | 7.2% |
| Japan | 6.4% |
The phage MuA transposase market demonstrates varied growth patterns across key countries, with China leading at an 11.5% CAGR through 2035, driven by massive biotechnology research investments, government support for genomics programs, and rapidly expanding pharmaceutical research capabilities. India follows at 10.6%, supported by growing biotechnology sector development, increasing research infrastructure investments, and expanding pharmaceutical industry capabilities.
Germany records 9.8% growth, emphasizing precision biotechnology research, advanced pharmaceutical development, and comprehensive molecular biology research programs. Brazil shows strong growth at 8.9%, expanding biotechnology research capabilities and pharmaceutical industry development.
The United States maintains 8.1% growth, focusing on advanced biotechnology innovation and pharmaceutical research leadership. The United Kingdom demonstrates 7.2% expansion, supported by genomics research excellence and biotechnology sector development. Japan records 6.4% growth, leveraging technological precision and advanced research capabilities.
The report covers an in-depth analysis of 40+ countries with top-performing countries highlighted below.
The phage MuA transposase market in China is projected to expand at the highest growth rate with a CAGR of 11.5% through 2035, driven by unprecedented biotechnology research investments, government support for genomics and precision medicine programs, and rapidly expanding pharmaceutical research and development capabilities.
The country's comprehensive biotechnology development strategy includes massive investments in research infrastructure, advanced laboratory facilities, and cutting-edge research programs that require sophisticated molecular biology tools including transposase enzymes. Major pharmaceutical companies and research institutions are establishing comprehensive research capabilities that support drug discovery, therapeutic development, and advanced genomics studies.
Government initiatives promoting biotechnology innovation are driving adoption of advanced research tools across academic institutions, pharmaceutical companies, and biotechnology startups. Leading international collaborations are facilitating technology transfer and knowledge sharing that supports advanced enzyme applications and research protocol development.
Revenue from phage MuA transposase in India is projected to grow at a CAGR of 10.6%, supported by expanding biotechnology sector development, increasing research infrastructure investments, and growing pharmaceutical industry capabilities across diverse therapeutic areas. The country's emphasis on biotechnology innovation and pharmaceutical manufacturing is driving demand for advanced molecular biology tools that support research applications, drug development programs, and comprehensive therapeutic studies.
Government programs promoting biotechnology research are creating favorable conditions for advanced research tool adoption and technology development. Major international companies are establishing research facilities that require comprehensive molecular biology capabilities and technical support services. Educational institutions and research organizations are developing technical expertise that supports advanced enzyme applications and research protocol implementation.
Demand for phage MuA transposase in Germany expanding at a CAGR of 9.8%, supported by the country's leadership in precision biotechnology research, advanced pharmaceutical development capabilities, and comprehensive molecular biology research programs. German research institutions and pharmaceutical companies are implementing sophisticated transposase applications that meet stringent quality standards while supporting complex research protocols and drug development programs.
The country's extensive pharmaceutical industry is driving significant transposase demand for advanced research applications and therapeutic development programs. Research institutions are collaborating with industry partners to develop next-generation research approaches that maintain German competitiveness in global biotechnology markets. Advanced manufacturing partnerships are facilitating technology development and knowledge sharing across research organizations and pharmaceutical companies.
The phage MuA transposase market in Brazil is growing at a CAGR of 8.9%, driven by expanding biotechnology research infrastructure, growing pharmaceutical industry capabilities, and increasing emphasis on advanced molecular biology research across academic and commercial sectors. Brazilian research institutions and pharmaceutical companies are investing in transposase tools to enhance research capabilities and support drug development programs while building domestic biotechnology expertise.
Government programs supporting biotechnology development are facilitating access to advanced research tools and technical expertise. Regional research centers are developing specialized capabilities that support transposase applications across pharmaceutical research, academic studies, and biotechnology development. International partnerships are providing technology transfer opportunities and technical assistance for advanced research implementations.
Demand for phage MuA transposase in the United States is anticipated to grow at a CAGR of 8.1%, driven by advanced biotechnology innovation programs, pharmaceutical research leadership, and comprehensive molecular biology research capabilities across academic and commercial sectors. American research institutions and pharmaceutical companies are implementing sophisticated transposase applications to maintain global competitiveness while supporting cutting-edge research programs and therapeutic development initiatives.
The biotechnology industry is driving significant transposase demand for advanced research applications, drug discovery programs, and comprehensive development protocols. Government initiatives supporting biotechnology research are creating substantial demand for advanced molecular biology tools and comprehensive technical support services. Regional research clusters are developing specialized expertise that supports diverse transposase applications and emerging research requirements.
The phage MuA transposase market in the United Kingdom is expected to expand at a CAGR of 7.2%, supported by genomics research excellence, biotechnology sector development, and increasing emphasis on advanced molecular biology research across diverse applications. British research institutions and biotechnology companies are investing in transposase solutions to support genomics studies, pharmaceutical research, and comprehensive biotechnology development while maintaining competitive research capabilities.
The country's strong academic research base is facilitating technology development and application innovation across diverse transposase requirements. Government initiatives supporting biotechnology research are creating favorable conditions for advanced research tool adoption and technology advancement. Advanced research partnerships are enabling knowledge sharing and technical collaboration that supports comprehensive transposase implementation across research applications.
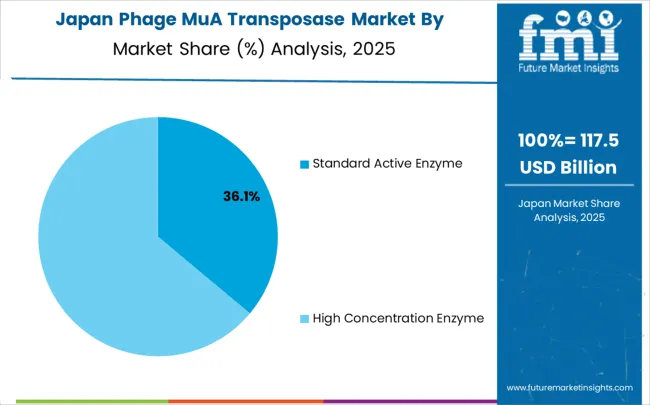
Revenue from phage MuA transposase in Japan is projected to expand at a CAGR of 6.4%, supported by technological precision capabilities, advanced research infrastructure, and emphasis on comprehensive molecular biology excellence across diverse scientific applications. Japanese research institutions and biotechnology companies are implementing sophisticated transposase solutions that demonstrate superior performance characteristics while supporting various research applications requiring exceptional precision and reliability.
The country's advanced biotechnology sector is driving demand for high-performance transposase enzymes that support precision research protocols and comprehensive quality requirements. Collaborative research programs between industry and academic institutions are developing innovative molecular biology approaches that maintain Japan's competitive advantage in global biotechnology markets.
Quality management systems and continuous improvement principles are driving adoption of transposase solutions that enhance research excellence and experimental reliability across diverse applications. Advanced research methodologies are enabling development of next-generation biotechnology approaches that provide enhanced research capabilities and comprehensive experimental validation.
The phage MuA transposase market in Europe is projected to expand from USD 86.7 million in 2025 to USD 196.1 million by 2035, registering a CAGR of 8.5% over the forecast period. Germany is expected to maintain its leadership with 33.8% market share in 2025, projected to grow to 34.3% by 2035, supported by its extensive biotechnology research infrastructure and pharmaceutical industry capabilities. France follows with 19.6% market share in 2025, expected to reach 19.9% by 2035, driven by genomics research programs and biopharmaceutical development activities.
The Rest of Europe region is projected to maintain stable share at 18.4% throughout the forecast period, attributed to emerging biotechnology research initiatives in Eastern European countries and expanding molecular biology research capabilities.
United Kingdom contributes 15.7% in 2025, projected to reach 15.3% by 2035, supported by advanced research institutions and biotechnology sector development. Italy maintains 12.5% share in 2025, expected to grow to 13.5% by 2035, while other European countries demonstrate steady growth patterns reflecting regional biotechnology development and research infrastructure advancement.
The phage MuA transposase market is characterized by competition among specialized biotechnology companies, molecular biology reagent manufacturers, and research tool providers. Companies are investing in advanced enzyme engineering, comprehensive quality control systems, application-specific formulations, and technical support services to deliver reliable, high-performance, and cost-effective transposase enzyme solutions. Strategic partnerships, technological innovation, and market expansion initiatives are central to strengthening product portfolios and research community presence.
Thermo Fisher Scientific, U.S.-based, provides comprehensive molecular biology reagent portfolios including transposase enzymes with emphasis on quality, reliability, and extensive application support across diverse research areas.
Sigma-Aldrich offers extensive enzyme portfolios with focus on quality assurance and comprehensive product support. New England Biolabs specializes in high-quality enzymes with comprehensive technical documentation and application support. Other key players including Promega, Bio-Rad Laboratories, Qiagen, Lucigen, Takara Bio, Invitrogen, Clontech, and Bio-Techne contribute specialized expertise and diverse technical capabilities across global research markets, providing comprehensive molecular biology solutions for various research applications.
Phage MuA transposase represents a critical molecular biology tool enabling precise DNA manipulation through transposition-based cloning, random mutagenesis, and genetic engineering applications. With the market expanding dramatically from $321.1M to $726.0M at 8.5% CAGR, driven by gene therapy advancement, synthetic biology growth, and personalized medicine development, this specialized enzyme market requires coordinated stakeholder action to address manufacturing scalability, application development, and regulatory pathway challenges.
Strategic Biotechnology Investment: Provide targeted funding for academic and industrial research utilizing MuA transposase in gene therapy development, synthetic biology applications, and vaccine research to maintain national competitiveness in critical biotechnology sectors.
Regulatory Science Enhancement: Invest in regulatory agencies' capacity to evaluate novel biotechnology applications using transposase systems, ensuring safety assessment capabilities keep pace with rapidly evolving genetic engineering technologies.
Biomanufacturing Infrastructure: Support development of domestic enzyme production capabilities including specialized fermentation facilities, purification systems, and quality control laboratories essential for biotechnology supply chain security.
International Collaboration Frameworks: Establish bilateral research agreements and technology sharing protocols that facilitate access to cutting-edge transposase applications while protecting intellectual property and maintaining technological advantages.
Skilled Workforce Development: Fund specialized training programs in molecular biology techniques, enzyme engineering, and biotechnology manufacturing to address the critical shortage of qualified personnel in rapidly expanding biotechnology sectors.
Fundamental Mechanism Research: Conduct advanced studies on MuA transposase structure-function relationships, substrate specificity, and engineering potential to enable next-generation enzyme variants with enhanced performance characteristics.
Application Development Initiatives: Lead collaborative projects exploring novel transposase applications in emerging fields including epigenome editing, organoid engineering, and precision agriculture that expand market opportunities.
Open Science Platforms: Contribute validated protocols, plasmid constructs, and application data to open repositories that accelerate research adoption while establishing institutional expertise and collaboration networks.
Cross-Disciplinary Integration: Foster collaborations between molecular biology, computational biology, and engineering disciplines to develop integrated transposase-based solutions for complex biotechnology challenges.
Technology Transfer Excellence: Establish robust mechanisms for translating academic transposase research into commercial applications through industry partnerships, licensing agreements, and startup formation.
Product Quality Excellence: Develop advanced purification and quality control processes that ensure consistent transposase activity, minimal contamination, and lot-to-lot reproducibility essential for critical research and therapeutic applications.
Application-Specific Optimization: Engineer specialized MuA transposase variants optimized for specific applications (high-throughput screening, mammalian cell engineering, plant transformation) rather than generic one-size-fits-all approaches.
Manufacturing Scale Innovation: Invest in scalable production platforms including high-density fermentation, automated purification, and advanced formulation technologies that enable cost-effective supply of growing market demand.
Technical Support Excellence: Provide comprehensive application support including protocol optimization, troubleshooting guidance, and custom assay development that creates customer dependency beyond simple product supply.
Cold Chain & Stability Optimization: Develop advanced enzyme stabilization and packaging technologies that extend shelf life, reduce storage requirements, and enable global distribution without performance degradation.
Integrated Platform Development: Create comprehensive genetic engineering platforms where MuA transposase functions as part of complete workflows including cloning, screening, and validation rather than isolated tools.
High-Throughput Application Focus: Develop automated systems and protocols that leverage transposase efficiency for large-scale genetic screens, library construction, and synthetic biology applications requiring massive parallelization.
Therapeutic Application Development: Advance transposase-based approaches for gene therapy delivery, CAR-T cell engineering, and vaccine development where precise genetic modification capabilities provide competitive advantages.
Diagnostic System Integration: Incorporate transposase-based amplification and detection methods into diagnostic platforms for infectious disease detection, genetic screening, and personalized medicine applications.
Custom Engineering Services: Offer specialized transposase engineering and optimization services for pharmaceutical and biotechnology clients requiring application-specific enzyme variants.
Drug Discovery Acceleration: Implement transposase-based genetic screening and target validation approaches that accelerate identification of therapeutic targets and mechanism-of-action studies for drug development programs.
Cell Line Development: Utilize MuA transposase for stable cell line generation, protein production optimization, and biomanufacturing platform development that reduces development timelines and costs.
Gene Therapy Applications: Explore transposase-based gene delivery systems as alternatives to viral vectors, particularly for applications requiring precise integration control and reduced immunogenicity.
Personalized Medicine Development: Integrate transposase-based genetic analysis and modification tools into precision medicine approaches including patient-derived organoids and companion diagnostic development.
Manufacturing Process Optimization: Apply transposase technology to optimize microbial and mammalian cell production systems for therapeutic proteins, vaccines, and other biotechnology products.
Biotechnology Innovation Investment: Finance R&D initiatives focused on next-generation transposase engineering, novel applications, and manufacturing process improvements that can significantly expand market opportunities.
Manufacturing Infrastructure Capital: Support investments in specialized enzyme production facilities, quality control laboratories, and distribution networks required to serve growing biotechnology markets globally.
Therapeutic Development Funding: Back companies developing transposase-based therapeutic approaches including gene therapies, cell therapies, and vaccine platforms that represent high-value market applications.
Platform Technology Investment: Finance integrated biotechnology platforms that incorporate transposase technology as part of comprehensive genetic engineering and synthetic biology solutions.
Global Market Expansion: Support international expansion initiatives that help transposase technology companies establish manufacturing and distribution capabilities in key growth markets.
Guidance Document Development: Create comprehensive regulatory guidance for transposase-based biotechnology applications including safety assessment requirements, quality standards, and approval pathways for therapeutic uses.
Scientific Advisory Integration: Establish expert panels including transposase researchers and biotechnology industry representatives to provide technical expertise for evaluating novel applications and emerging safety considerations.
International Harmonization: Collaborate with global regulatory agencies to develop consistent standards and approval pathways for transposase-based biotechnology products that facilitate international trade and development.
Risk Assessment Framework: Develop science-based approaches for evaluating safety and efficacy of transposase applications in different contexts from basic research tools to therapeutic interventions.
Innovation Pathway Programs: Implement expedited review processes for breakthrough transposase-based therapeutic approaches that address significant unmet medical needs while maintaining appropriate safety standards.
The phage MuA transposase market's rapid growth trajectory requires addressing the fundamental challenge of scaling specialized enzyme production while supporting diverse application development across academic research, biotechnology innovation, and therapeutic development, all within evolving regulatory frameworks for genetic engineering technologies.
| Item | Value |
|---|---|
| Quantitative Units (2025) | USD 321 million |
| Enzyme Type | Standard Active Enzyme, High Concentration Enzyme |
| End-Use Industry | Biopharmaceuticals, Vaccine Development, Functional Genomics Research, and Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, India, Brazil, Australia and 40+ countries |
| Key Companies Profiled | Thermo Fisher Scientific, Sigma-Aldrich, New England Biolabs, Promega, Bio-Rad Laboratories, Qiagen, Lucigen, Takara Bio, Invitrogen, Clontech, Bio-Techne |
| Additional Attributes | Dollar sales by enzyme type and end-use industry segments, regional demand trends across North America, Europe, and Asia-Pacific, competitive landscape with established molecular biology reagent manufacturers and specialized biotechnology companies, buyer preferences for standard versus high concentration enzyme formulations, integration with automated research workflows and high-throughput screening applications, innovations in enzyme engineering and application-specific solutions, and adoption of specialized formulations and comprehensive quality assurance systems for enhanced research reliability and experimental reproducibility. |
The global thin film platinum resistance temperature sensor market is estimated to be valued at USD 3,206.9 million in 2025.
The market size for the thin film platinum resistance temperature sensor market is projected to reach USD 5,907.7 million by 2035.
The thin film platinum resistance temperature sensor market is expected to grow at a 6.3% CAGR between 2025 and 2035.
The key product types in thin film platinum resistance temperature sensor market are pt100, pt500, pt1000 and others.
In terms of application, heating & air conditioning & ventilation segment to command 42.0% share in the thin film platinum resistance temperature sensor market in 2025.
Explore Similar Insights

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA