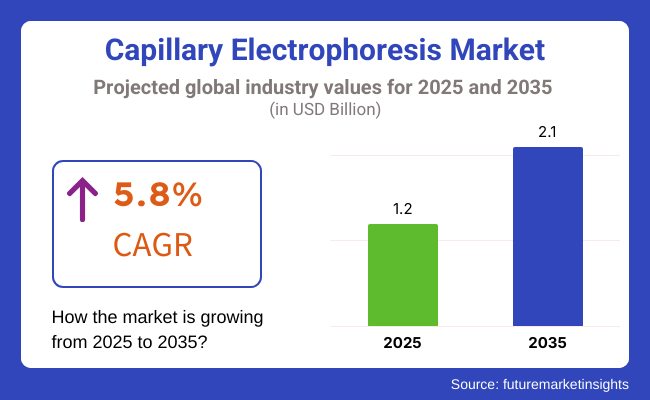
The capillary electrophoresis market is valued at USD 1.2 billion in 2025 and is expected to reach USD 2.1 billion by 2035, advancing at a 5.8 % CAGR.
North America remains the most lucrative region in 2025 thanks to deep biotech funding and widespread adoption of CE in DNA sequencing, while South Korea is forecast to be the fastest-growing country through 2035, propelled by its precision-medicine drive and heavy investment in biomanufacturing. CE’s ability to deliver high-resolution separations with minimal sample volumes keeps demand resilient across pharma QA/QC labs, academic cores, and forensic facilities.
Growth is powered by three converging forces: (1) soaring next-generation sequencing (NGS) throughput that needs orthogonal QC checks, (2) the shift toward complex biologics and oligonucleotide therapies where accurate purity profiling is non-negotiable, and (3) rising clinical-diagnostic use, from hemoglobinopathy screening to minimal-residual-disease (MRD) monitoring. Restraints include the high capital cost of multi-capillary instruments and a shortage of CE-trained analysts in emerging economies.
Key trends redefining the capillary electrophoresis market are microfluidic lab-on-chip devices, CE-MS hyphenation for intact protein analysis, and AI-driven peak deconvolution that slashes data-review times by 30%.
Between 2025 and 2035 the market will pivot from standalone bench systems to fully integrated, cloud-connected CE workstations. Vendors are embedding smart cartridges with RFID-tagged consumables that auto-log batch metadata, aiding 21 CFR Part 11 compliance. Portable CE analyzers-battery-powered and Wi-Fi enabled-will support point-of-care molecular diagnostics in remote clinics, while high-throughput platforms featuring 96-capillary arrays will boost NGS-library QC throughput by 4-fold.
Sustainability is gaining weight: solvent-free buffer chemistries and recyclable microfluidic chips are slated to cut per-test waste by up to 60 %. As personalized medicine scales, demand for ultra-precise charge-variant analysis and single-cell separations will sustain robust, single-digit growth, cementing CE as a core analytical backbone well into the next decade.
DNA sequencing and fragment analysis account for ~38 % of CE revenue in 2025, reflecting entrenched use in Sanger verification, microsatellite genotyping, and CRISPR off-target checks. Their popularity stems from CE’s unparalleled single-base resolution and automated sample tracking, which together guarantee submission-ready data for regulatory filings. Protein and peptide characterization follows closely (27 % share), with biopharma players relying on CE-SDS and cIEF to fingerprint monoclonal antibodies and gene-therapy capsids.
The dark-horse segment-drug impurity and stability profiling-is poised for the fastest expansion at a projected 7.0 % CAGR, fuelled by ICH Q6B and USP <729> directives that compel manufacturers to quantify charge variants and size heterogeneity in complex biologics.
Over the next decade, microfluidic CE-MS devices will marry sub-minute separations with high-resolution mass accuracy, shrinking analytical turnaround from days to hours. Meanwhile, forensic and environmental testing labs will adopt portable CE rigs for on-scene narcotics and contaminant screening, broadening the technology’s reach beyond traditional bench-top confines.
| Application Sub-segment | 2025 to 2035 CAGR |
|---|---|
| Drug Impurity & Stability Profiling | 7.0 % |
Pharmaceutical and biotech companies commanded roughly 49 % of 2025 CE sales, driven by regulatory mandates for rigorous biologic characterization and in-process control. These firms deploy multi-capillary systems alongside PAT (Process Analytical Technology) frameworks, enabling real-time release of vaccines, mAbs, and cell-therapy vectors. Academic and government research institutes represent 28 % of demand, leveraging CE for basic genomics, proteomics, and metabolomics research.
The real breakout lies in clinical diagnostics, projected to register a 6.5 % CAGR through 2035 as hospitals integrate CE-based assays for hemoglobin variant analysis, neonatal screening, and minimal residual disease detection in leukemia.
The convergence of CE with microfluidic cartridges and AI-guided interpretation will reduce hands-on time, allowing mid-tier labs to process 200-plus patient samples daily without specialist oversight. This democratization broadens access to high-precision separations in resource-constrained settings and secures a resilient growth runway for CE vendors.
| End-user Segment | 2025 to 2035 CAGR |
|---|---|
| Clinical Diagnostics | 6.5 % |
Challenges
High Cost of Advanced CE Systems The high cost of advanced capillary electrophoresis systems remains a challenge, as the capital required to develop advanced CE instruments is high; small research labs and diagnostic centers may not afford such systems. Lesser awareness in emerging trades:
There are certain regions of the developing countries where there still is no awareness and there are no trained professionals available, which are acting as hurdles that are hampering the acceleration of adoption. Additionally, as and when Regulatory Hurdles in Clinical Applications, the high implementation of electrophoresis-based diagnostic techniques in wide applications, including but not limited to healthcare and diagnostic, can face strict regulatory approval processes that can turn out to be a challenge for penetration.
Opportunities
CE Systems Technological Advancements are also driving the economy (including miniaturized, high-throughput, and automated electrophoresis systems), all of which have led to greater efficiency and affordability. These developments are enabling CE to be applied to an increasing number of laboratories and industries at lower operational costs and with enhanced analytical accuracy.
Another factor contributing to the demand is the expansion of use in drug development and quality control developments in systems and biomolecules. The increase in the use of CE in the pharmaceutical industry for drug formulation analysis, stability testing, and impurity profiling has led to the integration of this technique with commonly used workflows. The development and production of new drugs and biologics need reliable, fast, and reproducible analytical techniques, which drives this trend.
In the United States, the Capillary Electrophoresis industry is expanding as a result of growing demand for highly precise analytical techniques, rising applications in pharmaceutical research, and the invention of new biomolecular separation technology. The regulation of quality control by the Food and Drug Administration (FDA) is strict and encourages pharmaceutical and biotechnology companies to utilize capillary electrophoresis for drug development and quality control.
Capillary electrophoresis is being more and more widely used in academic research institutions and diagnostic laboratories for applications in DNA sequencing, proteomics, and forensic applications. AI-powered analytics and automation streamline the process of sample analysis, leading to increased efficiency and accuracy, consequently propelling economic growth.
Increasing implementation of field-to-laboratory analysis at the point of need and point of care is consistent with expanding the use of portable and high-throughput capillary electrophoresis devices. Key players such as Agilent Technologies, SCIEX, and Thermo Fisher Scientific are consistently launching new solutions designed to meet the shifting needs within the industry.
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 6.1% |
The UK Capillary Electrophoresis is influenced by a range of drivers including rising research activities in genomics, demand for accurate biomolecular analysis, and government support for innovations in life sciences. The UK Medicines and Healthcare Products Regulatory Agency (MHRA) corresponds with stringent practice for analytical methodologies, which propels the analytical approach demand for capillary electrophoresis systems.
The rising adoption of compact and space-saving electrophoresis instrumentation in laboratory and healthcare settings is driving the growth of the business.
| Country | CAGR (2025 to 2035) |
|---|---|
| UK | 5.8% |
The European Union Capillary Electrophoresis is growing owing to strict pharmaceutical regulations, increasing genomic research activities, and rising demand for precision analytical instruments. Users from all the leading pharmaceutical and biotechnology companies adopt the system as it complies with the stringent European Medicines Agency (EMA) guidelines for both drug characterization and biomolecular analysis.
The advanced capillary electrophoresis techniques are mainly used in Germany, France, and Italy for pharmaceutical research, clinical diagnostics, and proteomics. Harnessing AI for Data Analytics & Lab Automation AI is integrated with data analytics and laboratory automation to enhance the accuracy and efficiency of analytical workflows.
The industry is anticipated to grow due to a number of other factors, including the novel techniques of microfluidic capillary electrophoresis and high-throughput sequencing technologies, which enable the massive and precise molecular separations of the future in a matter of minutes across multiple industries.
| Region | CAGR (2025 to 2035) |
|---|---|
| European Union | 5.9% |
According to the report, factors such as supportive government initiatives, a surge in applications in clinical diagnostics, and advancements in separation science would drive the growth of the capillary electrophoresis in Japan during the given forecast period. In the pharmaceutical field, there is a good regulatory standard of analytical technique from the Japanese Ministry of Health, Labour, and Welfare, and there is high acceptance of using capillary electrophoresis in pharmaceutical quality control.
Capillary electrophoresis combined with AI-based analytics is being utilized by research organizations and healthcare providers for improved precision in genetic testing and diagnostics of disease. The growth is further being driven by automation and miniaturization of capillary electrophoresis instruments.
Global and regional countries: The growth for portable and high-resolution electrophoresis systems is growing, ensuring better and more efficient biomolecular analysis in different applications.
| Country | CAGR (2025 to 2035) |
|---|---|
| Japan | 6.0% |
Key drivers propelling the South Korea Capillary Electrophoresis business include increasing investments in biotechnology, escalating demand for precision medicine, and government progress towards life sciences innovation. This growing trend towards capillary electrophoresis in pharmaceutical and academic settings is, in large part, driven by the strict analytical testing standards set forth by the Ministry of Food and Drug Safety (MFDS).
In South Korea, AI-based electrophoresis analysis is widely adopted in industries to improve real-time molecular analysis and automated laboratory processes. The rising use of microfluidic capillary electrophoresis and high-throughput sequencing technologies will also continue to drive the growth in this country.
Increasing adoption with efficient accuracy and efficacy using smart laboratory solutions coupled with cloud-based data management is catalyzing the growth of the company for molecular diagnostics and biomolecular separations.
| Country | CAGR (2025 to 2035) |
|---|---|
| South Korea | 6.2% |
Agilent Technologies (18-23%)
Agilent leads the growth with cutting-edge CE platforms offering high sensitivity and automation, catering to pharmaceutical and biopharma research.
SCIEX (Danaher Corporation) (15 to 20%)
SCIEX is a key player in CE-MS applications, enabling advanced biomolecular characterization for clinical and proteomic studies.
Bio-Rad Laboratories (10-14%)
Bio-Rad focuses on specialized CE instruments for protein and nucleic acid analysis, widely used in research and forensic applications.
Thermo Fisher Scientific (8-12%)
Thermo Fisher provides robust and high-throughput CE solutions, integrating automation for improved workflow efficiency in analytical labs.
PerkinElmer Inc. (5-9%)
PerkinElmer offers cost-effective and compact CE systems designed for regulatory compliance and rapid separations in diverse applications.
Other Key Players (30-40% Combined)
Several additional companies contribute to the CE with innovations in sensitivity, speed, and application-specific systems. These include:
These companies continue to drive advancements in capillary electrophoresis technology, with a focus on automation, multi-capillary systems, and enhanced detection capabilities for various analytical and diagnostic applications.
The Global Capillary Electrophoresis industry is projected to witness a CAGR of 5.8% between 2025 and 2035.
USD 1.2 billion in 2025 was the worth of the Global Capillary Electrophoresis industry
The Global Capillary Electrophoresis industry is anticipated to reach USD 2.1 billion by 2035 end.
The highest CAGR is expected in North America due to the region's growing need for precise analytical methods and advances in genomics research.
The leading companies in the global capillary electrophoresis are Hoefer Inc., Helena Laboratories Corporation, Bio-Rad Laboratories, and Agilent Technologies.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Volume (Units) Forecast by Region, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 4: Global Market Volume (Units) Forecast by Application, 2018 to 2033
Table 5: Global Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 6: Global Market Volume (Units) Forecast by End User, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 8: North America Market Volume (Units) Forecast by Country, 2018 to 2033
Table 9: North America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 10: North America Market Volume (Units) Forecast by Application, 2018 to 2033
Table 11: North America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 12: North America Market Volume (Units) Forecast by End User, 2018 to 2033
Table 13: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Latin America Market Volume (Units) Forecast by Country, 2018 to 2033
Table 15: Latin America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 16: Latin America Market Volume (Units) Forecast by Application, 2018 to 2033
Table 17: Latin America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 18: Latin America Market Volume (Units) Forecast by End User, 2018 to 2033
Table 19: Western Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 20: Western Europe Market Volume (Units) Forecast by Country, 2018 to 2033
Table 21: Western Europe Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 22: Western Europe Market Volume (Units) Forecast by Application, 2018 to 2033
Table 23: Western Europe Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 24: Western Europe Market Volume (Units) Forecast by End User, 2018 to 2033
Table 25: Eastern Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 26: Eastern Europe Market Volume (Units) Forecast by Country, 2018 to 2033
Table 27: Eastern Europe Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 28: Eastern Europe Market Volume (Units) Forecast by Application, 2018 to 2033
Table 29: Eastern Europe Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 30: Eastern Europe Market Volume (Units) Forecast by End User, 2018 to 2033
Table 31: South Asia and Pacific Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 32: South Asia and Pacific Market Volume (Units) Forecast by Country, 2018 to 2033
Table 33: South Asia and Pacific Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 34: South Asia and Pacific Market Volume (Units) Forecast by Application, 2018 to 2033
Table 35: South Asia and Pacific Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 36: South Asia and Pacific Market Volume (Units) Forecast by End User, 2018 to 2033
Table 37: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 38: East Asia Market Volume (Units) Forecast by Country, 2018 to 2033
Table 39: East Asia Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 40: East Asia Market Volume (Units) Forecast by Application, 2018 to 2033
Table 41: East Asia Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 42: East Asia Market Volume (Units) Forecast by End User, 2018 to 2033
Table 43: Middle East and Africa Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 44: Middle East and Africa Market Volume (Units) Forecast by Country, 2018 to 2033
Table 45: Middle East and Africa Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 46: Middle East and Africa Market Volume (Units) Forecast by Application, 2018 to 2033
Table 47: Middle East and Africa Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 48: Middle East and Africa Market Volume (Units) Forecast by End User, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Application, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by End User, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 4: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 5: Global Market Volume (Units) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 9: Global Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 10: Global Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 11: Global Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 12: Global Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 13: Global Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 14: Global Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 15: Global Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 16: Global Market Attractiveness by Application, 2023 to 2033
Figure 17: Global Market Attractiveness by End User, 2023 to 2033
Figure 18: Global Market Attractiveness by Region, 2023 to 2033
Figure 19: North America Market Value (US$ Million) by Application, 2023 to 2033
Figure 20: North America Market Value (US$ Million) by End User, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 22: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 23: North America Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 26: North America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 27: North America Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 28: North America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 29: North America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 30: North America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 31: North America Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 34: North America Market Attractiveness by Application, 2023 to 2033
Figure 35: North America Market Attractiveness by End User, 2023 to 2033
Figure 36: North America Market Attractiveness by Country, 2023 to 2033
Figure 37: Latin America Market Value (US$ Million) by Application, 2023 to 2033
Figure 38: Latin America Market Value (US$ Million) by End User, 2023 to 2033
Figure 39: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 40: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 41: Latin America Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 45: Latin America Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 49: Latin America Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 50: Latin America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 52: Latin America Market Attractiveness by Application, 2023 to 2033
Figure 53: Latin America Market Attractiveness by End User, 2023 to 2033
Figure 54: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 55: Western Europe Market Value (US$ Million) by Application, 2023 to 2033
Figure 56: Western Europe Market Value (US$ Million) by End User, 2023 to 2033
Figure 57: Western Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 58: Western Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 59: Western Europe Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 60: Western Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 61: Western Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 62: Western Europe Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 63: Western Europe Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 64: Western Europe Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 65: Western Europe Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 66: Western Europe Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 67: Western Europe Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 68: Western Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 69: Western Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 70: Western Europe Market Attractiveness by Application, 2023 to 2033
Figure 71: Western Europe Market Attractiveness by End User, 2023 to 2033
Figure 72: Western Europe Market Attractiveness by Country, 2023 to 2033
Figure 73: Eastern Europe Market Value (US$ Million) by Application, 2023 to 2033
Figure 74: Eastern Europe Market Value (US$ Million) by End User, 2023 to 2033
Figure 75: Eastern Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 76: Eastern Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 77: Eastern Europe Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 78: Eastern Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 79: Eastern Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 80: Eastern Europe Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 81: Eastern Europe Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 82: Eastern Europe Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 83: Eastern Europe Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 84: Eastern Europe Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 85: Eastern Europe Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 86: Eastern Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 87: Eastern Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 88: Eastern Europe Market Attractiveness by Application, 2023 to 2033
Figure 89: Eastern Europe Market Attractiveness by End User, 2023 to 2033
Figure 90: Eastern Europe Market Attractiveness by Country, 2023 to 2033
Figure 91: South Asia and Pacific Market Value (US$ Million) by Application, 2023 to 2033
Figure 92: South Asia and Pacific Market Value (US$ Million) by End User, 2023 to 2033
Figure 93: South Asia and Pacific Market Value (US$ Million) by Country, 2023 to 2033
Figure 94: South Asia and Pacific Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 95: South Asia and Pacific Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 96: South Asia and Pacific Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 97: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 98: South Asia and Pacific Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 99: South Asia and Pacific Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 100: South Asia and Pacific Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 101: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 102: South Asia and Pacific Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 103: South Asia and Pacific Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 104: South Asia and Pacific Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 105: South Asia and Pacific Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 106: South Asia and Pacific Market Attractiveness by Application, 2023 to 2033
Figure 107: South Asia and Pacific Market Attractiveness by End User, 2023 to 2033
Figure 108: South Asia and Pacific Market Attractiveness by Country, 2023 to 2033
Figure 109: East Asia Market Value (US$ Million) by Application, 2023 to 2033
Figure 110: East Asia Market Value (US$ Million) by End User, 2023 to 2033
Figure 111: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 112: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 113: East Asia Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 114: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 115: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 116: East Asia Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 117: East Asia Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 118: East Asia Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 119: East Asia Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 120: East Asia Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 121: East Asia Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 122: East Asia Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 123: East Asia Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 124: East Asia Market Attractiveness by Application, 2023 to 2033
Figure 125: East Asia Market Attractiveness by End User, 2023 to 2033
Figure 126: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 127: Middle East and Africa Market Value (US$ Million) by Application, 2023 to 2033
Figure 128: Middle East and Africa Market Value (US$ Million) by End User, 2023 to 2033
Figure 129: Middle East and Africa Market Value (US$ Million) by Country, 2023 to 2033
Figure 130: Middle East and Africa Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 131: Middle East and Africa Market Volume (Units) Analysis by Country, 2018 to 2033
Figure 132: Middle East and Africa Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 133: Middle East and Africa Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 134: Middle East and Africa Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 135: Middle East and Africa Market Volume (Units) Analysis by Application, 2018 to 2033
Figure 136: Middle East and Africa Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 137: Middle East and Africa Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 138: Middle East and Africa Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 139: Middle East and Africa Market Volume (Units) Analysis by End User, 2018 to 2033
Figure 140: Middle East and Africa Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 141: Middle East and Africa Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 142: Middle East and Africa Market Attractiveness by Application, 2023 to 2033
Figure 143: Middle East and Africa Market Attractiveness by End User, 2023 to 2033
Figure 144: Middle East and Africa Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Capillary Underfill Material Market
Electrophoresis Reagents Market Size and Share Forecast Outlook 2025 to 2035
Electrophoresis Market Trends - Growth, Demand & Forecast 2025 to 2035
Electrophoresis Equipment and Supplies Market Analysis
Electrophoresis Transilluminator Market
Microchip Electrophoresis Market Size and Share Forecast Outlook 2025 to 2035
Sequencing Electrophoresis Systems Market Size and Share Forecast Outlook 2025 to 2035
Nucleic acid electrophoresis and blotting market
DNA Sequencing Electrophoresis Systems Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA