The global laminar airflow cabinet market is projected to grow from USD 214.3 million in 2025 to approximately USD 320.3 million by 2035, recording an absolute increase of USD 106.0 million over the forecast period. This translates into a total growth of 49.5%, with the market forecast to expand at a compound annual growth rate (CAGR) of 4.1% between 2025 and 2035. The market size is expected to grow by nearly 1.49X during the same period, supported by the rising adoption of advanced containment systems and increasing demand for sterile environments in pharmaceutical and healthcare sectors.
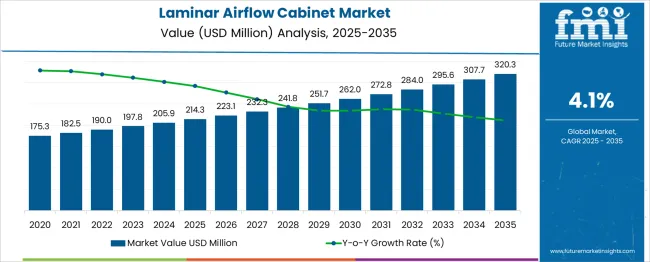
Between 2025 and 2030, the laminar airflow cabinet market is projected to expand from USD 214.3 million to USD 259.7 million, resulting in a value increase of USD 45.3 million, which represents 42.8% of the total forecast growth for the decade. This phase of growth will be shaped by rising demand for sterile work environments in pharmaceutical manufacturing, increasing investments in cleanroom technologies, and growing awareness about contamination control in critical applications. Service providers are expanding their product portfolios to address the growing complexity of modern laboratory requirements and regulatory compliance standards.
From 2030 to 2035, the market is forecast to grow from USD 259.7 million to USD 320.3 million, adding another USD 60.6 million, which constitutes 57.2% of the ten-year expansion. This period is expected to be characterized by expansion of automated airflow systems, integration of advanced filtration technologies, and development of energy-efficient cabinet designs. The growing adoption of biotechnology research and increasing focus on pharmaceutical quality assurance will drive demand for more sophisticated laminar airflow solutions and specialized containment systems.
Between 2020 and 2025, the laminar airflow cabinet market experienced steady expansion, driven by increasing pharmaceutical research activities and growing awareness of contamination control requirements in laboratory environments. The market developed as research facilities and manufacturing units recognized the need for specialized equipment to maintain sterile conditions during critical processes. Regulatory authorities and quality assurance teams began emphasizing proper containment procedures to maintain product safety and compliance standards.
| Metric | Value |
|---|---|
| Estimated Value in 2025E | USD 214.3 million |
| Forecast Value in 2035F | USD 320.3 million |
| Forecast CAGR (2025 to 2035) | 4.1% |
Market expansion is being supported by the rapid increase in pharmaceutical research and development activities worldwide and the corresponding need for sterile work environments in drug manufacturing and testing facilities. Modern laboratories rely on precise airflow control and HEPA filtration to ensure contamination-free conditions for sensitive processes including cell culture work, pharmaceutical compounding, and quality testing procedures. Even minor contamination events can require complete batch disposal and comprehensive facility decontamination to maintain regulatory compliance.
The growing complexity of biotechnology applications and increasing regulatory requirements are driving demand for professional-grade laminar flow cabinets from certified manufacturers with appropriate safety certifications and performance validation. Regulatory agencies are increasingly requiring documented airflow verification and particle counting procedures following equipment installation to maintain facility accreditation and ensure process integrity. Quality standards and pharmaceutical manufacturing guidelines are establishing standardized containment procedures that require specialized equipment and trained operation protocols.
The market is segmented by size outlook, product outlook, class outlook, end-use outlook, and region. By size outlook, the market is divided into 4x2 feet, 2x2 feet, 3x2 feet, 6x2 feet, 8x2 feet, and others. Based on product outlook, the market is categorized into vertical laminar flow cabinets and horizontal laminar flow cabinets. In terms of class outlook, the market is segmented into Class 1, Class 2, and Class 3. By end-use outlook, the market is classified into pharmaceutical facilities, food & beverage, hospitals, semiconductor & electronics, and cleanrooms. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
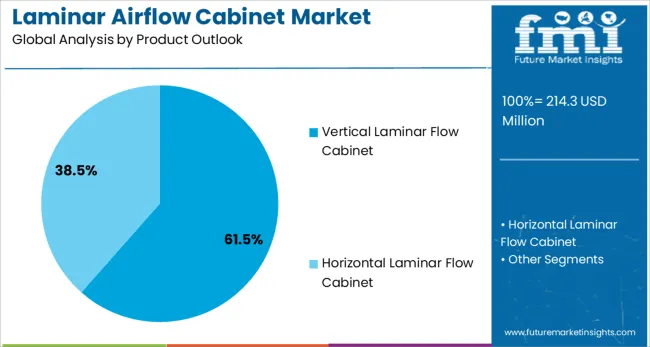
Vertical laminar flow cabinets are projected to account for 61.5% of the Laminar Airflow Cabinet market in 2025. This leading share is supported by the widespread adoption of vertical airflow systems for personnel protection applications and sterile product handling in pharmaceutical manufacturing environments. Vertical flow cabinets provide superior operator safety through downward airflow patterns that direct contaminated air away from the work surface and user breathing zone. The segment benefits from established safety protocols and comprehensive equipment availability from multiple certified manufacturers.
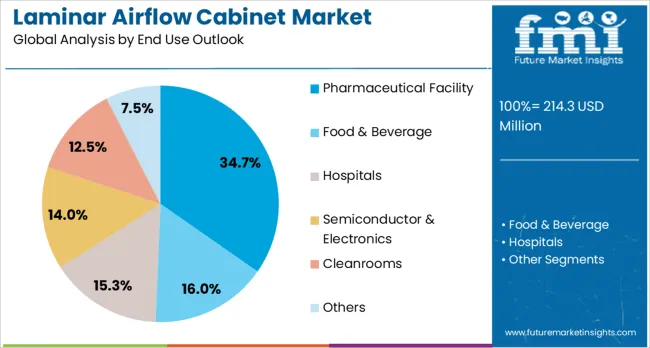
Pharmaceutical facilities are expected to represent 34.7% of laminar airflow cabinet demand in 2025. This dominant share reflects the critical importance of sterile environments in drug manufacturing and quality control testing procedures. Modern pharmaceutical operations increasingly require multiple containment systems to support diverse manufacturing processes including sterile compounding, aseptic filling, and analytical testing activities. The segment benefits from stringent regulatory requirements for contamination control and increasing investments in facility modernization programs.
The Laminar Airflow Cabinet market is advancing steadily due to increasing pharmaceutical research activities and growing recognition of contamination control importance. The market faces challenges including high equipment acquisition costs, need for continuous maintenance and filter replacement, and varying performance requirements across different application areas. Standardization efforts and certification programs continue to influence product development and market expansion patterns.
The growing deployment of energy-efficient laminar flow systems is enabling reduced operational costs while maintaining superior contamination control performance. Modern cabinets equipped with variable speed motors and intelligent airflow monitoring systems provide optimized energy consumption without compromising safety standards. These technologies are particularly valuable for facilities with multiple cabinet installations that require consistent performance with reduced utility expenses and environmental impact.
Modern laminar flow cabinet manufacturers are incorporating advanced airflow monitoring equipment and automated control systems that improve performance reliability and reduce maintenance requirements. Integration of real-time particle counting systems and digital airflow verification enables continuous performance validation and comprehensive documentation for regulatory compliance. Advanced monitoring also supports predictive maintenance scheduling and automated filter replacement indicators.
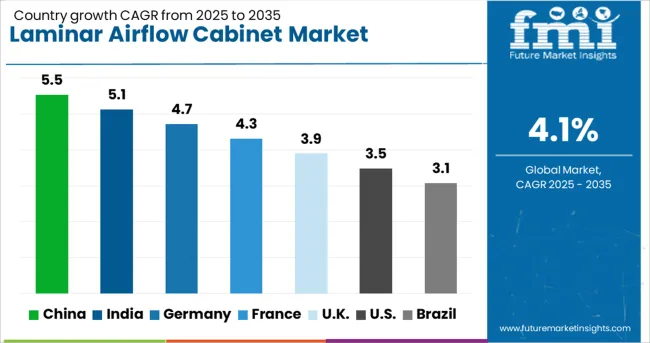
| Countries | CAGR (2025-2035) |
|---|---|
| China | 5.5% |
| India | 5.1% |
| Germany | 4.7% |
| France | 4.3% |
| UK | 3.9% |
| USA | 3.5% |
| Brazil | 3.1% |
The laminar airflow cabinet market demonstrates varied growth patterns across key regions, with China leading at 5.5% CAGR through 2035, driven by expanding pharmaceutical manufacturing and increasing laboratory infrastructure investments. India follows at 5.1%, supported by growing biotechnology sector development and regulatory compliance requirements. Germany records 4.7% growth, emphasizing precision manufacturing and advanced containment technologies, while France shows 4.3% expansion through research facility modernization programs.
Revenue from laminar airflow cabinets in China is projected to exhibit the highest growth rate with a CAGR of 5.5% through 2035, driven by rapid expansion of pharmaceutical manufacturing facilities and increasing investments in biotechnology research infrastructure. The country's growing pharmaceutical export industry and domestic drug development programs are creating significant demand for sterile manufacturing environments and contamination control systems. Major pharmaceutical companies and research institutes are establishing comprehensive cleanroom facilities to support both traditional drug manufacturing and advanced biotechnology applications.
Government pharmaceutical industry development programs are supporting establishment of world-class manufacturing facilities that meet international quality standards and regulatory requirements for global market access. Pharmaceutical manufacturing modernization initiatives are facilitating adoption of advanced containment technologies that enhance product quality assurance and operational efficiency across major industrial centers.
Revenue from laminar airflow cabinets in India is expanding at a CAGR of 5.1%, supported by increasing pharmaceutical manufacturing activities and growing biotechnology research investments. The country's expanding generic drug production industry and increasing focus on pharmaceutical quality assurance are driving demand for sterile work environments and contamination control systems. Research institutions and pharmaceutical manufacturers are gradually establishing comprehensive cleanroom capabilities to serve both domestic and international market requirements.
Pharmaceutical industry growth and biotechnology sector development are creating opportunities for specialized containment system providers that can support diverse manufacturing processes and regulatory compliance standards. Professional training and equipment certification programs are developing technical expertise among facility operators, enabling comprehensive contamination control procedures that meet international pharmaceutical manufacturing standards.
Demand for laminar airflow cabinets in Germany is projected to grow at a CAGR of 4.7%, supported by the country's focus on pharmaceutical manufacturing excellence and precision engineering applications. German pharmaceutical facilities are implementing comprehensive contamination control systems that meet stringent quality standards and regulatory requirements for high-value drug manufacturing processes. The market is characterized by a focus on technological innovation, advanced equipment integration, and compliance with comprehensive pharmaceutical manufacturing regulations.
Pharmaceutical manufacturing investments prioritize advanced containment technologies that demonstrate superior performance reliability while meeting German pharmaceutical quality and safety standards. Professional certification programs ensure comprehensive technical expertise among facility operators, enabling specialized contamination control procedures that support diverse pharmaceutical manufacturing applications and regulatory compliance requirements.
Revenue from laminar airflow cabinets in France is growing at a CAGR of 4.3%, driven by ongoing pharmaceutical research facility upgrades and increasing biotechnology sector investments. The country's established pharmaceutical industry and growing focus on specialized drug development are creating demand for advanced containment systems and sterile work environments. Research institutions and pharmaceutical manufacturers are investing in modern laboratory infrastructure to support innovative drug development programs and maintain competitive positioning.
Pharmaceutical research industry modernization facilitates adoption of advanced containment technologies that support comprehensive contamination control capabilities across major research and manufacturing centers. Professional development programs enhance technical capabilities among facility operators, enabling specialized sterile environment maintenance procedures that meet evolving pharmaceutical industry requirements.
Demand for laminar airflow cabinets in the UK is expanding at a CAGR of 3.9%, driven by continued pharmaceutical research excellence and biotechnology sector growth. The country's established research institutions and pharmaceutical companies are maintaining comprehensive containment capabilities to support advanced drug development programs and specialized manufacturing processes. The market benefits from strong regulatory framework requirements and a focus on pharmaceutical quality assurance standards.
Pharmaceutical research investments prioritize advanced containment system capabilities that support innovative drug development and biotechnology applications across major research centers. Professional training programs develop specialized expertise among laboratory operators, enabling comprehensive sterile environment management that supports world-class pharmaceutical research and development activities.
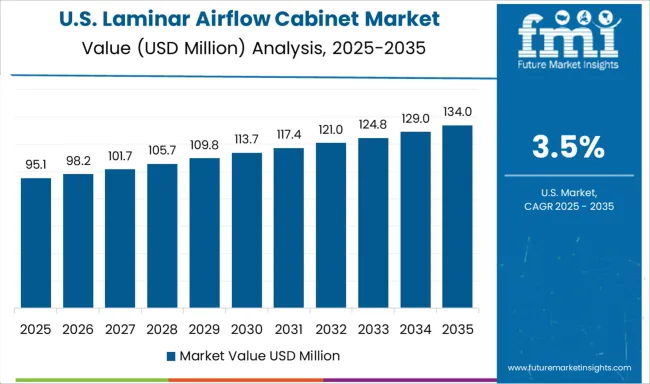
Demand for laminar airflow cabinets in the United States is expanding at a CAGR of 3.5%, driven by ongoing pharmaceutical facility upgrades and continued research institution investments. The country's established pharmaceutical industry and mature biotechnology sector maintain consistent demand for containment systems through facility modernization programs and regulatory compliance requirements. Major pharmaceutical companies and research organizations are implementing advanced contamination control systems to support both traditional drug manufacturing and specialized biotechnology applications across diverse therapeutic areas.
Pharmaceutical industry consolidation and research facility optimization programs are creating opportunities for advanced containment system providers that offer comprehensive installation and maintenance services. Professional certification programs ensure technical expertise among facility operators, enabling specialized contamination control procedures that meet FDA regulatory standards and pharmaceutical manufacturing guidelines. The market benefits from established supply chain networks and comprehensive technical support infrastructure that facilitates equipment deployment and ongoing operational support.
Revenue from laminar airflow cabinets in Brazil is growing at a CAGR of 3.1%, supported by expanding pharmaceutical manufacturing activities and increasing focus on quality assurance standards. The country's developing pharmaceutical industry and growing generic drug production sector are creating demand for sterile manufacturing environments and contamination control systems. Pharmaceutical manufacturers and research facilities are gradually establishing comprehensive cleanroom capabilities to serve domestic market requirements and potential export opportunities.
Pharmaceutical industry development programs facilitate adoption of international quality standards that require proper contamination control procedures and certified equipment installations. Professional training initiatives develop technical expertise among facility operators, enabling comprehensive sterile environment management that meets pharmaceutical manufacturing standards and regulatory compliance requirements. The market benefits from government support for pharmaceutical industry modernization and increasing focus on domestic drug production capabilities.
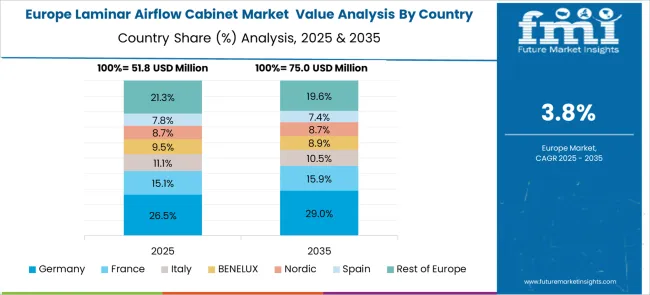
The laminar airflow cabinet market in Europe is projected to grow steadily over the forecast period, with established pharmaceutical manufacturing centers driving consistent demand for containment systems. Germany is expected to maintain market leadership supported by its extensive pharmaceutical industry infrastructure and research facility investments. The European market demonstrates a strong focus on advanced pharmaceutical quality standards and comprehensive contamination control technologies, particularly in pharmaceutical manufacturing applications and research laboratory requirements.
European laminar airflow cabinet demand patterns show concentration in high-performance applications, with vertical laminar flow cabinets and Class 2 systems representing dominant product segments across major markets. The region's strict pharmaceutical quality codes and regulatory standards drive preference for certified contamination control equipment, supporting premium technology adoption and specialized supplier relationships. Germany's pharmaceutical and biotechnology clusters, France's pharmaceutical manufacturing applications, and regional supply chain integration continue to strengthen European market position in global pharmaceutical equipment and contamination control system applications.
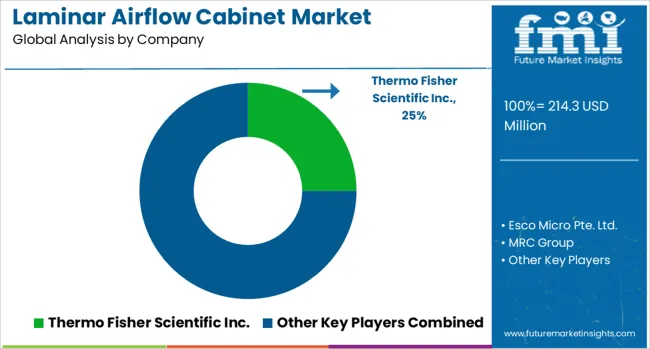
The laminar airflow cabinet market is defined by competition among specialized contamination control equipment manufacturers, laboratory equipment companies, and pharmaceutical technology providers. Companies are investing in advanced filtration technologies, production processes, contamination control materials, and comprehensive technical support to deliver precise, reliable, and cost-effective airflow cabinet solutions. Strategic partnerships, technological innovation, and geographic expansion are central to strengthening service portfolios and market presence.
Major players in the market include Thermo Fisher Scientific Inc., known for comprehensive laboratory equipment solutions and global service support networks. Esco Micro Pte. Ltd. offers specialized containment systems with a focus on pharmaceutical and biotechnology applications. MRC Group provides advanced cleanroom technologies and contamination control equipment for diverse industrial applications. Brinda Pharma Technologies emphasizes pharmaceutical manufacturing solutions, while Bionics Scientific Technologies focuses on precision laboratory equipment and sterile work environments.
Additional market participants include Labocon, Roch Mechatronics Inc., Biobase Biodusty, Lamsystems, Airclean Systems, Allentown Inc., Kimberly-Clark Corporation, Labconco Corporation, and NuAire, each contributing specialized expertise in contamination control and laboratory equipment technologies.
| Items | Values |
|---|---|
| Quantitative Units | USD 320.34 million |
| Size Outlook | 4x2 Feet, 2x2 Feet, 3x2 Feet, 6x2 Feet, 8x2 Feet, and Others |
| Product Outlook | Vertical Laminar Flow Cabinet, Horizontal Laminar Flow Cabinet |
| Class Outlook | Class 1, Class 2, Class 3 |
| End Use Outlook | Pharmaceutical Facility, Food & Beverage, Hospitals, Semiconductor & Electronics, Cleanrooms |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, India, China, United Kingdom, Japan, Brazil |
| Key Companies Profiled | Thermo Fisher Scientific Inc., Esco Micro Pte. Ltd., MRC Group, Brinda Pharma Technologies, Bionics Scientific Technologies (P) Ltd., Labocon, Roch Mechatronics Inc., Biobase Biodusty (Shandong), Co., Ltd., Lamsystems, Airclean Systems, Allentown Inc., Kimberly-Clark Corporation, Labconco Corporation, NuAire |
| Additional Attributes | Dollar sales by size outlook, product outlook, class outlook, and end-use outlook, regional demand trends across North America, Europe, and Asia-Pacific, competitive landscape with established manufacturers and emerging technology providers, facility preferences for energy-efficient versus conventional systems, integration with digital monitoring and control platforms, innovations in filtration technology and airflow optimization systems, and adoption of smart cabinet solutions with embedded sensors, performance monitoring, and automated maintenance scheduling for enhanced operational efficiency. |
The global laminar airflow cabinet market is estimated to be valued at USD 214.3 million in 2025.
The market size for the laminar airflow cabinet market is projected to reach USD 320.3 million by 2035.
The laminar airflow cabinet market is expected to grow at a 4.1% CAGR between 2025 and 2035.
The key product types in laminar airflow cabinet market are 4x2 feet, 2x2 feet, 3x2 feet, 6x2 feet and 8x2 feet.
In terms of product outlook, vertical laminar flow cabinet segment to command 61.5% share in the laminar airflow cabinet market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Laminar Airflow Trolley Market Size and Share Forecast Outlook 2025 to 2035
Interlaminar Device Market
Airflow Balancer Market Size and Share Forecast Outlook 2025 to 2035
Banquet Cabinets Market – Storage & Heating Solutions 2025-2035
Display Cabinets Market
Medicine Cabinets Market Size and Share Forecast Outlook 2025 to 2035
Bathroom Cabinets Market Size and Share Forecast Outlook 2025 to 2035
Industrial Drying Cabinets Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Cable Distribution Cabinets Market Size and Share Forecast Outlook 2025 to 2035
Medical Smart Drug Cabinet Market Size and Share Forecast Outlook 2025 to 2035
Holding and Proofing Cabinets Market - Temperature Control & Bakery Solutions 2025 to 2035
Banquet Carts and Heated Cabinets Market
Stainless Steel Electrical Cabinet Market Size and Share Forecast Outlook 2025 to 2035
Energy-saving Constant Humidity Storage Cabinet Market Size and Share Forecast Outlook 2025 to 2035
Fully Sealed Fully Insulated Inflatable Cabinet Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA