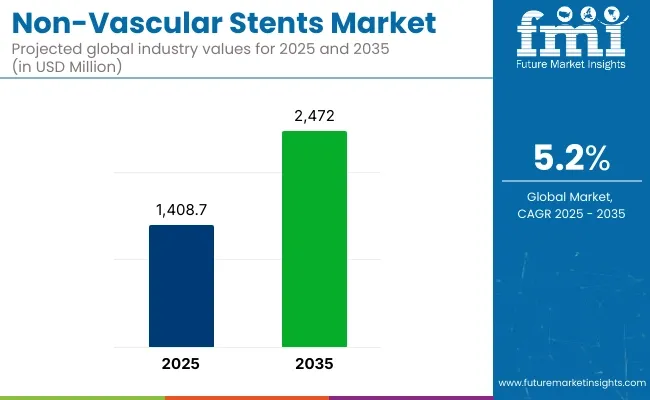
The global Non-Vascular Stents Market is estimated to be valued at USD 1,408.7 million in 2025 and is projected to reach USD 2,472.0 million by 2035, registering a compound annual growth rate (CAGR) of 5.2% over the forecast period.
The non-vascular stents market is expanding steadily as minimally invasive therapies gain traction across multiple specialties, including gastroenterology, pulmonology, urology, and oncology. Growing demand for palliative interventions in malignant obstructions, as well as treatment of benign strictures, is driving procedure volumes globally.
Advancements in stent design, such as drug-eluting coatings, fully covered metal stents, and biodegradable platforms, are addressing limitations associated with restenosis, migration, and long-term complications. Increasing incidence of gastrointestinal cancers, chronic obstructive pulmonary diseases, biliary obstructions, and benign prostatic hyperplasia is fueling clinical adoption.
As healthcare systems increasingly prioritize minimally invasive approaches to reduce healthcare costs and improve patient quality of life, the non-vascular stents market is positioned for steady growth, particularly with emerging bioabsorbable and next-generation polymer-based stent technologies entering late-stage development.
Key manufacturers such as Boston Scientific, Cook Medical, Taewoong Medical, Olympus Corporation, Micro-Tech, and Merit Medical are actively expanding their non-vascular stent portfolios. These companies are focusing on advanced materials, improved anti-migration designs, and drug-eluting technologies to improve both short- and long-term patient outcomes. In 2024, CGBIO’s Biliary Stent ‘ARISTENT’ Receives PMDA Approval in Japan “Accelerating Global Expansion Starting with Japanese Market.
Hyun-seung Yu, CEO of CGBIO, stated, “Following the launch of the biliary ARISTENT, we plan to sequentially release stents for the esophagus, duodenum, and colon to strengthen our competitiveness. We will expand our business in the gastroenterology field through synergy with CG gel, a gastrointestinal hemostatic powder to be released in the future.
Through this, we aim to emerge as a global stent specialist company and solidify our position in the global medical device market by accelerating our entry into global markets such as the USA and Europe, starting with a domestic launch in the near future.” The launch addresses the growing demand for stents tailored to advanced endoscopic interventions, particularly in oncology-driven GI procedures.
North America dominates the non-vascular stents market, supported by high procedural volumes, early adoption of advanced endoscopic techniques, and payer reimbursement for minimally invasive interventions.
USA tertiary hospitals are rapidly adopting fully covered metal stents and endoscopic ultrasound-guided stenting techniques in complex GI malignancies. Growing collaborations between oncologists and interventional endoscopists are driving wider adoption of palliative stenting for advanced-stage cancers.
Europe’s non-vascular stents market is expanding, driven by national healthcare system cost-efficiency measures and growing procedural expertise in endoscopic and bronchoscopic stenting. Germany, France, and the Nordics are leading adoption of advanced covered metal stents for esophageal, biliary, and airway applications.
EU MDR regulatory harmonization is facilitating CE mark clearances for new biodegradable stent platforms. Public payers increasingly support minimally invasive stenting procedures as alternatives to surgery for high-risk patients.
| Attributes | Values |
|---|---|
| Estimated Size, 2025 | USD 1,408.7 million |
| Projected Size, 2035 | USD 2,472.0 million |
| Value-based CAGR (2025 to 2035) | 5.2% |
The below table presents the expected CAGR for the global non-vascular stents market over several semi-annual periods spanning from 2025 to 2035. In the first half (H1) of the decade from 2024 to 2035, the business is predicted to surge at a CAGR of 5.9%, followed by a slightly lower growth rate of 5.6% in the second half (H2) of the same decade.
| Particular | Value CAGR |
|---|---|
| H1 (2024 to 2034) | 5.9% |
| H2 (2024 to 2034) | 5.6% |
| H1 (2025 to 2035) | 5.2% |
| H2 (2025 to 2035) | 4.7% |
Moving into the subsequent period, from H1 2025 to H2 2035, the CAGR is projected to decrease slightly to 5.2% in the first half and decrease moderately at 4.7% in the second half. In the first half (H1) the market witnessed a decrease of 70.00 BPS while in the second half (H2), the market witnessed an increase of 90.00 BPS.
In 2025, ureteral stents are expected to hold 38.7% of the revenue share in the overall non-vascular stents market. This dominance is driven by the increasing prevalence of urological conditions, such as kidney stones, urinary tract obstructions, and ureteral strictures, which require ureteral stents to maintain urine flow and relieve obstruction.
The growth of this segment has been fueled by advancements in stent designs that offer improved comfort, reduced risk of infection, and easier placement. Ureteral stents are preferred due to their relatively simple insertion process and their critical role in preventing complications like kidney damage and infection. Additionally, the increasing number of patients undergoing minimally invasive urological surgeries, as well as rising awareness regarding kidney health, has further propelled the adoption of ureteral stents.
In 2025, non-metallic stents are projected to hold 52.2% of the revenue share in the non-vascular stents market. This segment's leadership is attributed to the growing preference for biocompatible materials that reduce the risk of adverse reactions and improve patient comfort.
Non-metallic stents, such as those made from silicone, polyurethane, and biopolymers, are increasingly favored for their flexibility, ease of insertion, and reduced risk of tissue irritation compared to traditional metal stents. The growth of this segment has been driven by advancements in materials science, which have enhanced the performance and longevity of non-metallic stents.
Furthermore, non-metallic stents are often preferred in sensitive areas, such as the ureter and bile ducts, where minimizing foreign body reactions is crucial. The rising adoption of non-metallic stents is also supported by the increasing shift towards minimally invasive procedures and the demand for stents that are easier to remove and less likely to cause complications, making them a popular choice in many medical settings.
P1: The global Non-Vascular Stents Market is reported to reach a valuation of USD 1,408.7 million in 2025 to USD 2,472.0 million by 2035. Demand for Non-Vascular Stents is expected to rise at a CAGR of 5.2% over the forecast period.
Ureteral Stents is expected to hold a 38.7% share in 2025, which is attributed to driven by the increasing prevalence of urological conditions. Non-Metallic Stents will dominate the application segment with a 52.2% market share, owing to the growing preference for biocompatible materials that reduce the risk of adverse reactions and improve patient comfort.
Innovations in Product Design and Materials Is Driving the Growth for Non-Vascular Stents Market
Advanced materials involving nitinol, cobalt-chromium, and polymer composites have revolutionized the stent durability, flexibility, and biocompatibility. These result in better adaptability to the anatomical structure, reduced risks of migration or perforation, and comfort for the patient.
For example, nitinol’s shape-memory property allows stents to expand upon placement and conform to dynamic anatomical changes, particularly in gastrointestinal or biliary applications.
Additionally, novel designs such as fully covered stents and self-expanding stents have improved the treatment of strictures and obstructions. Fully covered stents prevent tissue ingrowth, reducing complications like restenosis, while self-expanding stents enhance procedural precision and adaptability in complex cases. These innovations address the limitations of traditional designs, driving broader adoption among healthcare providers.
An example is the WallFlex Biliary RX Stent System by Boston Scientific, which combines a self-expanding nitinol structure with a fully covered design. This stent effectively manages biliary obstructions caused by malignancies while reducing migration risks. Another example is the Zilver PTX Stent by Cook Medical, which integrates nitinol with drug-eluting technology, ensuring both structural support and localized drug delivery.
In summary, continuous advancements in materials and design improve stent efficacy and safety, fueling the expansion of the non-vascular stents market.
Advancement In Delivery Systems Is a Significant Driver for The Growth of the Non-Vascular Stents Market
Advanced delivery systems facilitate smooth stent placement. It simplifies the process of stent placement and contributes to reducing complexity and probability of error. This hydrophilic property reduces friction while passing through, and it ensures less trauma to the patient during the process.
Radiopaque markers provide accurate vision by ensuring that surgeons visualize their pathways under imaging, thereby facilitating ideal positioning during complex anatomical locations.
Advances in this direction reduce the likelihood of complications, decrease procedure time, and better overall patient outcomes; therefore, the use of non-vascular stents is the preferred option by both healthcare providers and patients.
This ease of use and the enhanced safety profile has greatly increased the adoption of non-vascular stents for the treatment of biliary, esophageal, and colonic obstructions.
Examples of innovation in delivery system design include Boston Scientific's WallFlex Biliary RX Stent System, offering a controlled release delivery mechanism designed for precise placement, and the Hanarostent Esophagus by M.I. Tech, which delivers through a distinct delivery system devised for smooth implantation in an esophageal stricture. These systems prove how advanced technology is tailored specifically to meet needs in clinical life.
In conclusion, minimally invasive deployment technologies not only enhance the usability and effectiveness of non-vascular stents but also reduce procedural risks, contributing to their growing adoption and driving market expansion globally.
Drug-Eluting Stents Which Release Therapeutic Agents Locally Are Gaining Traction in The Non-Vascular Stents Market
Drug-eluting non-vascular stents are the most promising in terms of the market opportunity as they serve the dual purpose of maintaining luminal patency and delivering therapeutic agents directly to the site of obstruction.
These stents release drugs locally, which minimizes systemic side effects and effectively addresses inflammation, restenosis, or infections associated with malignant and benign obstructions. This localized drug delivery is particularly advantageous for patients with malignancies, where tumor progression can be mitigated alongside the mechanical relief of blockages.
One of the examples is the Cook Medical Zilver PTX Drug-Eluting Peripheral Stent. It is developed to treat blockages in the biliary duct. This drug-eluting stent releases paclitaxel, which is a drug known for its anti-proliferative properties and reduces the chance of restenosis while allowing bile flow to be maintained. Its proven efficacy in challenging conditions has positioned it as a leader in the biliary stent market.
An example of this is the Boston Scientific WallFlex Biliary RX Fully Covered Stent System with Anti-Reflux Valve, a drug-eluting mechanism designed for malignant biliary strictures. It prevents the re-narrowing of bile ducts while incorporating anti-reflux technology that improves the outcomes of the patients and reduces complications.
In summary, drug-eluting non-vascular stents present a lucrative opportunity in the market, driven by their advanced therapeutic capabilities and effectiveness in managing complex conditions. Their dual-action mechanism positions them as a transformative solution in interventional medicine.
Risk of Infection and Biofilm Formation Emerging as Significant Growth Barrier for Non-Vascular Stents Market Growth
Infection and biofilm formation pose a serious barrier to growth in the non-vascular stents market. Infection related to the placement of a stent can lead to serious complications such as extended hospitalization, reoperation, and increased health costs. These risks might keep both healthcare providers and patients away from stent-based treatments, hence limiting the market potential.
Additionally, particular concern is biofilm formation, which involves the attachment of bacteria to the surface of the stent, multiplication, and the formation of a protective layer that is quite hard to eradicate.
This phenomenon increases not only the chances of infection but also complicates the treatment outcomes. Biofilms have been shown to form on stents rapidly, especially in those that have been in place for a long time, leading to chronic infections and other complications.
Moreover, heightened awareness of such risks might make healthcare providers more prudent in the prescription of non-vascular stents, especially in sensitive patient groups. Such prudence might delay the use of new stent technologies, even while new technologies have been developed to reduce infection risks.
Moreover, the higher rate of complications will also invite stricter regulatory requirements for the approval of new products, increasing the time and further costs of bringing new stent solutions to the market.
Thus, it becomes difficult for the manufacturer to develop effective and safe products that could address these issues while remaining economically viable for widespread use. Therefore, the double threats of infection and biofilm formation substantially hinder the growth trajectory of the non-vascular stents market.
Tier 1 companies comprise market leaders with a significant market share of 58.9% in global market. These companies engage in strategic partnerships and acquisitions to expand their product portfolios and access cutting-edge technologies.
Additionally, they emphasize extensive clinical trials to validate the efficacy and safety of their products. Prominent companies in tier 1 include Boston Scientific Corporation, Becton, Dickinson and Company (BD), Medtronic and Cook Medical.
Tier 2 companies include mid-size players having presence in specific regions and highly influencing the local market and holds around 21.9% market share. They typically pursue partnerships with multispecialty hospitals and research organizations to leverage emerging technologies and expedite product development.
These companies often emphasize agility and adaptability, allowing them to quickly bring new products to market, additionally targeting specific types medical needs. Additionally, they focus on cost-effective production methods to offer competitive pricing. Prominent companies in tier 2 include CONMED Corporation, ELLA - CS, s.r.o., Glaukos Corporation and HOBBS MEDICAL, INC.
Finally, Tier 3 companies, such as Micro-Tech (Nanjing) Co., Ltd. and Merit Medical Systems. They specialize in specific products and cater to niche markets, adding diversity to the industry.
Overall, while Tier 1 companies are the primary drivers of the market, Tier 2 and 3 companies also make significant contributions, ensuring the non-vascular stents sales remains dynamic and competitive.
The section below covers the industry analysis for the non-vascular stents market for different countries. Market demand analysis on key countries in several regions of the globe, including North America, Latin America, East Asia, South Asia & Pacific, Western Europe, Eastern Europe and Middle Ease & Africa, is provided.
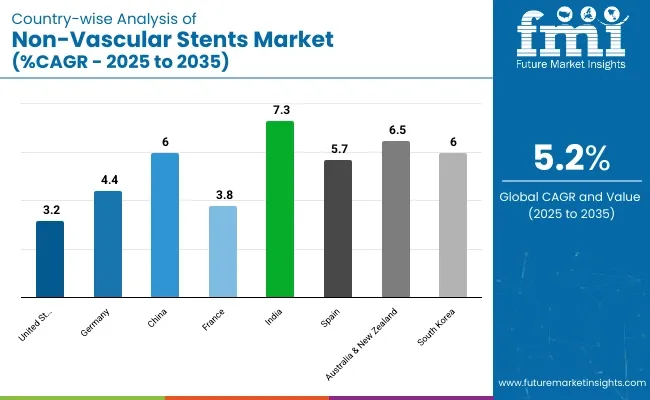
The United States is anticipated to remain at the forefront in North America, with higher market share through 2035. In South Asia & Pacific, India is projected to witness a CAGR of 7.3% by 2035.
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| United States | 3.2% |
| Germany | 4.4% |
| China | 6.0% |
| France | 3.8% |
| India | 7.3% |
| Spain | 5.7% |
| Australia & New Zealand | 6.5% |
| South Korea | 6.0% |
United States. non-vascular stents market is poised to exhibit a CAGR of 3.2% between 2025 and 2035. Currently, it holds the highest share in the North American market.
Companies with robust market presence like Medtronic, Boston Scientific, and Cook Medical dominate the market share and enhance growth prospects of non-vascular stents in the USA market.
Due to this heavy investment on research and development (R&D) activity, new and improved technologies of products enhance its position among consumers and have positive implications for better patient outcome as far as advanced technologies, drug-eluting and biodegradable stent.
The other reason is that these companies exploit their huge distribution networks and strong relationships with health care providers for widespread acceptance of their products. The competitive landscape forces constant innovation and fast reaction to the demands of the market, which will help the environment flourish for new technologies.
The USA market reaps the advantages of diverse, quality non-vascular stents meeting the increased demand for minimally invasive procedures to address several chronic conditions effectively. This interaction of innovation with the market consolidates the position of the USA at the top in the market for non-vascular stents.
China’s non-vascular stents market is poised to exhibit a CAGR of 6.0% between 2025 and 2035. Currently, it holds the highest share in the East Asia market, and the trend is expected to continue during the forecast period.
A high geriatric population in China is the key growth driver in the non-vascular stents market. Aged patients are more likely to be diagnosed with conditions like gastrointestinal, biliary, and tracheobronchial obstructions that necessitate the application of non-vascular stents for treatment.
The population change has seen the increased demand for minimally invasive procedures among aged patients, especially since these treatments are characterized by less time for recovery and a decreased risk of surgical complications.
China's healthcare landscape is transforming itself to meet the needs of the aging population and has been seen to provide ample access to more diagnostic and therapeutic technologies.
There is an ever-increasing rise in age-related diseases, resulting in heavy investment in medical infrastructure to make all these technologies readily available and affordable. This results in a high geriatric demographic in China becoming a significant enabler for growth, not just in terms of adoption but also innovation of non-vascular stents in China.
India non-vascular stents market is poised to exhibit a CAGR of 7.3% between 2025 and 2035. Currently, it holds the highest share in the South Asia & Pacific market, and the trend is expected to continue during the forecast period.
The increasing burden of diseases that require stent implantation is one of the key drivers for the non-vascular stents market in India. The increasing prevalence of gastrointestinal obstructions, biliary tract blockages, and urinary disorders increases the need for effective treatment options.
Such factors include dietary habits, high obesity levels, and an increasing percentage of geriatric population lead to a rising trend in such diseases, which further calls for the use of non-vascular stents to reduce symptoms and improve patient outcome.
The treatment preference now shifts toward less invasive, non-vascular stents as it results in short recovery periods with lower complication risk, mainly in chronic diseases. Availability of the diagnostic equipment across all hospitals and health facilities further improves the detection of obstructive diseases in early stages, further adding to demand.
The high prevalence of diseases within the population also has been found to provide huge market potential hence this parameter drives up the use and manufacturing of non-vascular stents throughout India.
The market players are using strategies to stay competitive, such as product differentiation through innovative formulations, strategic partnerships with healthcare providers for distribution.
Another key strategic focus of these companies is to actively look for strategic partners to bolster their product portfolios and expand their global market presence.
Recent Industry Developments in Non-Vascular Stents Market
In terms of product type, the industry is divided into biliary stents, esophageal stents, colonic stents, bronchial stents, and ureteral stents.
In terms of material, the industry is segmented into metal stents, non-metallic stents, and hybrid stents.
In terms of application, the industry is segmented into malignant obstructions, benign obstructions, and palliative care.
In terms of technology, the industry is segmented into self-expanding stents, balloon-expandable stents, drug-eluting stents, biodegradable/bioresorbable stents, and anti-reflux stents.
In terms of end user, the industry is segmented into hospitals, specialty clinics, and ambulatory surgical centers (ASCs).
Key countries in North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, and the Middle East and Africa (MEA) are covered in the report.
The global non-vascular stents market is projected to witness CAGR of 5.2% between 2025 and 2035.
The global non-vascular stents industry stood at USD 1,339.1 million in 2024.
The global non-vascular stents market is anticipated to reach USD 2,338.8 million by 2035 end.
India is set to record the highest CAGR of 7.3% in the assessment period.
The key players operating in the global non-vascular stents market include Boston Scientific Corporation, Becton, Dickinson and Company (BD), Medtronic, Cook Medical, CONMED Corporation, ELLA - CS, s.r.o., Glaukos Corporation, HOBBS MEDICAL, INC, Micro-Tech (Nanjing) Co., Ltd. and Merit Medical Systems.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Venous Stents Market Size and Share Forecast Outlook 2025 to 2035
Enteral Stents Market
Coronary Stents Market Insights – Trends, Growth & Forecast 2025-2035
Nephrology Stents and Catheters Market
Bioabsorbable Stents Market Growth – Trends & Forecast 2025 to 2035
Nonvascular Interventional Radiology Device Market Trends – Growth & Forecast 2024-2034

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA