The peripheral angioplasty Market is valued at USD 3.66 billion in 2025. As per FMI's analysis, the industry will grow at a CAGR of 7.7% and reach USD 7.7 billion by 2035. The peripheral angioplasty industry is expected to witness robust growth over the next decade, driven by the rising incidence of peripheral artery disease (PAD), increasing geriatric population, and growing demand for minimally invasive procedures.
Technological advancements in angioplasty devicessuch as drug-coated balloons and next-generation stentsare expected to improve patient outcomes and reduce recurrence, thus supporting long-term industry expansion.
Favorable reimbursement policies and expanding healthcare infrastructure, especially in emerging economies, will further boost the adoption of peripheral angioplasty. North America will maintain its dominance due to early technology adoption and high prevalence of PAD, while Asia Pacific is projected to register the fastest growth owing to improving diagnostics and rising health awareness.
| Metric | Value |
|---|---|
| Industry Value (2025E) | USD 3.66 billion |
| Industry Value (2035F) | USD 7.7 billion |
| CAGR (2025 to 2035) | 7.7% |
The peripheral angioplasty industry is on a sustained growth trajectory, driven by rising global prevalence of peripheral artery disease and a strong shift toward minimally invasive treatment options. Technological innovation in balloon catheters and stents is enhancing procedure efficacy and long-term patient outcomes. Stakeholders who invest in R&D, outpatient service expansion, and emerging industries stand to gain the most, while those slow to adopt innovation risk losing industry share.
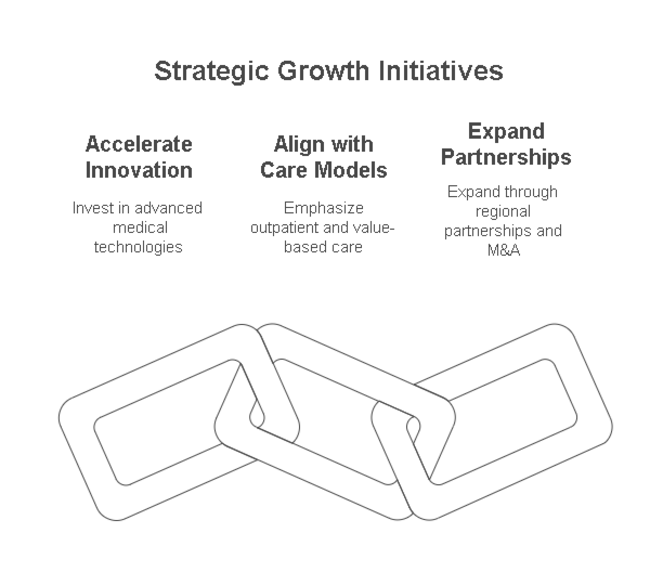
Accelerate Innovation in Device Technology
Invest in the development of next-generation drug-coated balloons and bioresorbable scaffolds to increase procedural success and reduce restenosis.
Align with Shifting Care Models
Focus on outpatient care expansion and value-based care models that emphasize faster recovery and cost efficiency.
Expand Through Partnerships and Regional Penetration
Pursue strategic partnerships with regional hospitals and clinics in Asia-Pacific and Latin America; consider M&A for tech integration and faster industry entry.
| Risk | Probability - Impact |
|---|---|
| Supply chain disruptions for catheter components | Medium - High |
| Regulatory delays in emerging industries | High - Medium |
| Rising competition from alternative PAD therapies | Medium - Medium |
| Priority | Immediate Action |
|---|---|
| Develop regional go-to- industry plan | Run feasibility on expanding in APAC and Latin America |
| Advance catheter innovation | Initiate OEM feedback loop on drug-coated balloon designs |
| Strengthen channel incentives | Launch aftermarket channel partner incentive pilot |
The next decade in peripheral angioplasty will be defined by innovation, outpatient care models, and global industry penetration. Companies must prioritize device enhancement and invest in fast-growing regions to capture upcoming demand shifts.
Leadership should recalibrate their strategic roadmap to integrate agile R&D cycles, forge ecosystem partnerships, and scale value-based service delivery. Acting now will secure long-term positioning as a front-runner in the next phase of PAD treatment evolution.
(Surveyed Q4 2024, n=450 stakeholder participants evenly distributed across medtech manufacturers, hospital procurement heads, interventional cardiologists, and health insurance firms in the USA, Western Europe, India, China, and Japan)
Regional Variance
High Variance
ROI Perspectives
Consensus
Regional Variance
Shared Challenges
Regional Differences
Manufacturers
Hospitals/Procurement Heads
Alignment
Divergence
High Consensus
Key Variances
Strategic Insight
| Countries/Region | Policy & Regulatory Impact |
|---|---|
| United States | CMS expanded reimbursement for outpatient PAD treatments, boosting procedural volume in ambulatory surgical centers (ASCs). - FDA requires 510(k) clearance or PMA for devices; DCBs and bioresorbable scaffolds need clinical efficacy data. - HIPAA and interoperability policies apply to AI-integrated systems. |
| Western Europe (EU) | EU MDR (Medical Device Regulation) has increased documentation and clinical evidence requirements, delaying industry entry for new angioplasty tools. - CE Mark is mandatory for all devices. - Green compliance regulations encourage use of recyclable materials and low-impact packaging. |
| India | CDSCO oversees angioplasty device approvals under the Medical Devices Rules, 2017. - Importers must obtain registration certificates; local trials increasingly required. - No universal reimbursement framework yet, creating pricing pressure in public hospitals. |
| China | NMPA mandates localized clinical trials for high-risk Class III devices like angioplasty catheters and DCBs. - Government favors domestic manufacturers via procurement policies under the “Made in China 2025” strategy. - GHTF compliance gaining traction. |
| Japan | PMDA regulates angioplasty devices under the Pharmaceuticals and Medical Devices Act (PMD Act). - Shonin approval needed, with stringent safety and efficacy data. - Cost control policies affect high-end device adoption; reimbursement revisions occur every 2 years. |
Boston Scientific CorporationEstimated Industry Share: ~20-25%
A global leader with a strong portfolio in drug-coated balloons (DCBs) and stents. Its Ranger™ DCB and Innova™ stent system dominate several international industries.
Medtronic plcEstimated Industry Share: ~18-22%
Offers an extensive range of peripheral vascular solutions including the IN.PACT™ Admiral DCB, widely used for PAD. Maintains high industry penetration in North America and Europe.
Abbott LaboratoriesEstimated Industry Share: ~15-18%
A major player with innovations like the Omnilink Elite™ Vascular Balloon-Expandable Stent. Strong growth in Asia-Pacific due to regulatory clearances and pricing flexibility.
BD (Becton, Dickinson and Company)Estimated Industry Share: ~10-12%
Focused on cost-effective PTA balloon catheters. Gains traction in emerging industries due to high-volume manufacturing and supply chain optimization.
Cook MedicalEstimated Industry Share: ~8-10%
Known for its niche portfolio of peripheral intervention devices. Focuses on physician training and direct hospital partnerships for industry expansion.
Cardinal Health (Cordis)Estimated Industry Share: ~5-7%
Gains industry share in Latin America and parts of Asia with accessible peripheral stent systems. Strong distribution networks drive reach.
BiotronikEstimated Industry Share: ~3-5%
European-based firm with a selective but innovative peripheral device lineup. Focuses on research-driven growth and regional partnerships.
The balloon catheters are expected to dominate the peripheral angioplasty industry with a CAGR of 5.2% from 2025 to 2035. The balloon catheters are expected to remain a foundational component of peripheral angioplasty procedures due to their minimally invasive nature and effectiveness in treating stenotic lesions. Advancements in drug-coated balloon technologies are expected to enhance clinical outcomes, particularly in cases of recurrent peripheral arterial disease.
Stents, especially self-expanding and drug-eluting variants, will likely see sustained demand owing to their ability to reduce restenosis and provide long-term vessel patency. While innovation in stent design has plateaued in recent years, manufacturers are now focusing on bioresorbable stents and hybrid solutions that combine mechanical support with pharmacological benefits.
Guidewires will also maintain steady growth throughout the forecast period, as these devices are essential for navigating complex vascular anatomy during angioplasty. The rise in chronic lifestyle diseases, coupled with improved operator training and procedural volumes in emerging industries, will drive sustained adoption across all three product categories.
The hospitals segments are expected to dominate the peripheral angioplasty industry with a CAGR of 6.3% from 2025 to 2035. Hospitals will continue to dominate the end-user segment of the peripheral angioplasty industry between 2025 and 2035, largely due to their established infrastructure, multidisciplinary care offerings, and ability to manage complex vascular cases. Tertiary hospitals and government-run medical centers will also benefit from increased public sector investment in cardiovascular health infrastructure across both developed and developing regions.
Ambulatory surgical centers are poised for robust expansion during the same period, as payers and patients increasingly prioritize lower-cost, outpatient alternatives for routine endovascular procedures. These centers benefit from faster turnaround times and reduced overheads, making them attractive venues for elective angioplasties.
Cardiac catheterization centers will see growing utilization in urban healthcare ecosystems, particularly in North America and Europe, where cardiovascular screening programs are routinely integrated into preventive care. These centers offer high procedural efficiency, advanced imaging modalities, and shorter patient recovery times-factors that align well with the shift toward value-based vascular interventions.
The United States peripheral angioplasty industry is anticipated to grow with a CAGR of 7.9% in the forecast period (2025 to 2035). This growth is attributed to a rise in incidents of peripheral artery disease (PAD), especially in the geriatric section and the use of diabetes and obesity.
Broad insurance coverage for angioplasty procedures by Medicare and commercial payers will remain supportive of procedural volumes. Plus, the move to outpatient is driving investments in ambulatory surgical centers and cardiac catheterization laboratories.
The USA also has a strong R&D ecosystem, with big players including Boston Scientific and Medtronic releasing next-generation drug-coated balloons and stents. The FDA’s 510(k) and PMA pathways, which provide regulatory stability while giving companies the opportunity to bring new ideas to industry without obtaining their approval too slowly, is also a large factor in the US’s growing position in this space.
Demand for peripheral angioplasty in the United Kingdom is expected to rise at a CAGR of around 6.9% during 2025 to 2035. This is increasingly being recognized by the National Health Service (NHS) as a crucial area of focus in the Long-Term Plan for Cardiovascular Disease Management (CVD), which, in turn, is helping to raise awareness and improve the early diagnosis of PAD.
Despite a continued hospital-centric approach with procedural care in the UK, some incremental adoption has taken place with outpatient angioplasty services being established in a few urban based centers. However, some friction still exists for importing devices, albeit with benefits for key players from the ease of regulation alignment via the UKCA marking process post-Brexit.
Venture funding also has been complemented by government investments in the fields of digital health and tele-cardiology that have further enabled more personalized treatment pathways. With the presence of clinical registries and multi-center research programs, the UK has undoubtedly become a valuable refuge for the evaluation of new endovascular technologies and procedural best practices.
The French Peripheral Angioplasty Industry is expected to grow at a CAGR of around 6.8% during the forecast period. As the country has a highly centralized public health system with widespread access to PAD diagnosis and intervention services, the demand for peripheral vascular procedures is kept high. In France, where minimal invasive techniques are also much appreciated, drug-eluting stents and balloon catheters are extremely popular.
Via the French Social Security Financing Law, this plan classified angioplasty in chronic care plans simplifying the reimbursement mechanisms to local players and multinational corporations. French hospitals are also among both participants in European clinical trials, as well as the European device performance benchmarking, to provide strong data for product efficacy. Investments in hybrid operating rooms and vascular specialist training forge a powerful ecosystem in which procedural growth can continue to thrive.
Germany Peripheral Angioplasty Industry is anticipated to grow at a steady CAGR of 7.5% during the forecast period. Germany is at the forefront of Europe regarding procedural volume and technology because of its decentralized healthcare structure and many specialized vascular centers. Strong public-private partnerships help procure advanced medical devices, and patients enjoy relatively short wait times for PAD diagnosis and treatment.
Drug-coated balloon angioplasty has been embraced by German hospitals, where many of the clinical trials used to set wider EU device guidelines originate. The country’s health insurers are very much on board with minimally invasive interventions, especially in older populations. Local manufacturers and regional distributors have strong relationships with university hospitals, accelerating early technology rollouts and product feedback cycles.
The peripheral angioplasty industry in Italy is anticipated to be growing at a compound annual growth rate (CAGR) of 6.6% during the forecast period. Italy has also improved its vascular infrastructure in recent years, particularly in Northern Italy, even though the country’s healthcare system is publicly funded and regionally governed. More investments in vascular diagnostic tools as well as intervention capabilities are being observed due to growing prevalence of diabetes, smoking and hypertension leading to increased PAD bioburden.
Training programs supported by European vascular societies and integration of hybrid catheterization labs in tertiary centers across Italy. New peripheral angioplasty devices are more slowly adopted than in Germany and France, but also more and more local physicians join multicenter studies exploring long-term results. Although reimbursement remains a struggle, device companies are targeting metropolitan areas for higher-margin procedures.
The peripheral angioplasty industry in New Zealand is likely to grow at a CAGR of 6.1% between 2025 and 2035. New Zealand faces increasing cases of PAD, especially among its Māori and Pasifika populations, who show higher incidences of diabetes and lifestyle-related risk factors. However, procedural access remains uneven between urban and rural populations due to centralized vascular facilities.
The public health system covers most angioplasty procedures but long wait times can delay timely intervention. The government is gradually expanding investments in cardiovascular infrastructure through regional health boards.
Private hospital networks and telehealth collaborations are beginning to facilitate faster diagnostics. Device penetration remains low compared to Australia, but New Zealand’s close regulatory alignment with Australian standards helps streamline device approval and procurement.
The peripheral angioplasty industry in South Korea is forecast to expand at a CAGR of 7.6% through 2035. South Korea’s rapid technological adoption, combined with a strong national health insurance system, has led to increased demand for vascular interventions. Urban hospitals, especially in Seoul and Busan, offer advanced catheterization labs with imaging integration and robotic assistance.
Local manufacturers are also entering the angioplasty device space, supported by government funding through the Ministry of Health and Welfare’s innovation programs. PAD screening initiatives have expanded under the national preventive health plan, encouraging earlier-stage intervention.
Additionally, collaborations with global medical device firms are fueling faster access to high-end drug-eluting balloons and next-gen guidewires, supporting South Korea’s emergence as a regional hub for peripheral vascular care.
The peripheral angioplasty industry in Japan is projected to grow at a CAGR of 6.4% over the 2025 to 2035 period. Despite its aging population and advanced medical infrastructure, Japan exhibits a conservative approach to adopting new angioplasty devices due to high regulatory scrutiny under the PMDA. However, once approved, technologies see high uptake in academic medical centers.
The government continues to invest in preventive cardiovascular health, and angioplasty procedures for PAD are fully reimbursed under national insurance, creating favorable economics for high-volume hospitals.
Japan is also leading in hybrid operating room installations and robotic-assisted vascular procedures. While drug-coated balloons are gradually gaining traction, Japanese clinicians still favor long-term efficacy data before widespread adoption. Local manufacturers are developing cost-effective stent systems that meet both quality and price expectations.
China's peripheral angioplasty industry is expected to grow at a CAGR of 8.2% from 2025 to 2035, the highest among major economies. This surge is driven by rising lifestyle diseases and government focus on expanding access to vascular procedures under its “Healthy China 2030” initiative. Tier 1 and Tier 2 cities are witnessing the establishment of advanced vascular intervention centers, while rural penetration remains limited but is improving.
Domestic manufacturers benefit from government procurement incentives and are rapidly closing the quality gap with foreign brands. The National Medical Products Administration (NMPA) has streamlined clinical trial approvals, accelerating the introduction of drug-eluting and bioresorbable devices.
International companies are partnering with local firms to navigate the regulatory environment and capture volume-driven growth. China’s growing healthcare infrastructure and middle-class expansion support long-term angioplasty demand.
The peripheral angioplasty industry in Australia is forecast to grow at a CAGR of 6.7% from 2025 to 2035. Australia maintains a dual public-private healthcare structure that allows both Medicare-covered and out-of-pocket angioplasty procedures.
The country has seen steady growth in PAD awareness, thanks to national health campaigns and targeted cardiovascular screening programs. Most angioplasty procedures are concentrated in metropolitan regions such as Sydney and Melbourne, although telehealth expansion is improving rural access.
Australian hospitals are among the earliest adopters of global innovations, often piloting next-gen balloon catheters and stents under post-industry surveillance agreements. Regulatory efficiency under the Therapeutic Goods Administration (TGA) encourages quick but safe device adoption. Continued investment in cardiovascular research and skilled labor training will support the industry's upward trajectory through 2035.
The industry is segmented into balloon catheters, stents, and guidewires.
The industry is segmented into hospitals, ambulatory surgical centers, and cardiac catheterization centers.
The industry is segmented into North America, Latin America, Europe, East Asia, South Asia, Oceania, and the Middle East & Africa.
The peripheral angioplasty industry is expected to reach approximately USD 7.7 billion by 2035, growing at a CAGR of 7.7% from 2025 to 2035.
Asia Pacific, particularly China and India, is anticipated to exhibit the highest growth due to increasing PAD prevalence, improved healthcare spending, and favorable government initiatives.
Key drivers include the rising burden of lifestyle diseases, advancements in minimally invasive technologies, and growing awareness among patients and healthcare professionals about early diagnosis and treatment of PAD.
Major players include Medtronic, Boston Scientific, Abbott, Terumo, and BD, all of which have a strong presence globally through innovative product lines and strategic partnerships.
Regulatory frameworks such as FDA approvals in the USA and CE marking in Europe are critical for product commercialization. These regulations ensure product safety, efficacy, and promote trust among healthcare providers and patients.
Table 01: Global Market Value (US$ Million) Analysis and Forecast 2012 to 2022, By Product
Table 02: Global Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 03: Global Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 04: Global Market Value (US$ Million) Analysis and Forecast 2023 to 2033, by Region
Table 05: North America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 06: North America Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 07: North America Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 08: North America Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 09: Latin America Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 10: Latin America Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 11: Latin America Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 12: Latin America Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 13: Europe Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 14: Europe Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 15: Europe Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 16: Europe Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 17: South Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 18: South Asia Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 19: South Asia Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 20: South Asia Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 21: East Asia Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 22: East Asia Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 23: East Asia Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 24: East Asia Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 25: Oceania Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 26: Oceania Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 27: Oceania Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 28: Oceania Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Table 29: Middle East & Africa Market Value (US$ Million) Analysis 2012 to 2022 and Forecast 2023 to 2033, by Country
Table 30: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By Product
Table 31: Middle East & Africa Market Volume (Units) Analysis and Forecast 2023 to 2033, By Product
Table 32: Middle East & Africa Market Value (US$ Million) Analysis and Forecast 2023 to 2033, By End User
Figure 01: Global Market Volume (Units), 2012 to 2022
Figure 02: Global Market Volume (Units) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 03: Pricing Analysis per unit (US$), in 2022
Figure 04: Pricing Forecast per unit (US$), in 2033
Figure 05: Global Market Value (US$ Million) Analysis, 2012 to 2022
Figure 06: Global Market Forecast & Y-o-Y Growth, 2023 to 2033
Figure 07: Global Market Absolute $ Opportunity (US$ Million) Analysis, 2023 to 2033
Figure 08: Global Market Value Share (%) Analysis 2023 and 2033, By Product
Figure 09: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By Product
Figure 10: Global Market Attractiveness Analysis 2023 to 2033, By Product
Figure 11: Global Market Value Share (%) Analysis 2023 and 2033, By End User
Figure 12: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By End User
Figure 13: Global Market Attractiveness Analysis 2023 to 2033, By End User
Figure 14: Global Market Value Share (%) Analysis 2023 and 2033, by Region
Figure 15: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, by Region
Figure 16: Global Market Attractiveness Analysis 2023 to 2033, by Region
Figure 17: North America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 18: North America Market Value (US$ Million) Forecast, 2022 to 2032
Figure 19: North America Market Value Share, By Product (2023 E)
Figure 20: North America Market Value Share, By End User (2023 E)
Figure 21: North America Market Value Share, by Country (2023 E)
Figure 22: North America Market Attractiveness Analysis By Product, 2023 to 2033
Figure 23: North America Market Attractiveness Analysis By End User, 2023 to 2033
Figure 24: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 25: USA Market Value Proportion Analysis, 2022
Figure 26: Global Vs. USA Growth Comparison
Figure 27: USA Market Share Analysis (%) By Product, 2022 & 2032
Figure 28: USA Market Share Analysis (%) By End User, 2022 & 2032
Figure 29: Canada Market Value Proportion Analysis, 2022
Figure 30: Global Vs. Canada. Growth Comparison
Figure 31: Canada Market Share Analysis (%) By Product, 2023 & 2033
Figure 32: Canada Market Share Analysis (%) By End User, 2023 & 2033
Figure 33: Latin America Market Value (US$ Million) Analysis, 2012 to 2022
Figure 34: Latin America Market Value (US$ Million) Forecast, 2022 to 2032
Figure 35: Latin America Market Value Share, By Product (2023 E)
Figure 36: Latin America Market Value Share, By End User (2023 E)
Figure 37: Latin America Market Value Share, by Country (2023 E)
Figure 38: Latin America Market Attractiveness Analysis By Product, 2023 to 2033
Figure 39: Latin America Market Attractiveness Analysis By End User, 2023 to 2033
Figure 40: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 41: Mexico Market Value Proportion Analysis, 2022
Figure 42: Global Vs Mexico Growth Comparison
Figure 43: Mexico Market Share Analysis (%) By Product, 2023 & 2033
Figure 44: Mexico Market Share Analysis (%) By End User, 2023 & 2033
Figure 45: Brazil Market Value Proportion Analysis, 2022
Figure 46: Global Vs. Brazil. Growth Comparison
Figure 47: Brazil Market Share Analysis (%) By Product, 2023 & 2033
Figure 48: Brazil Market Share Analysis (%) By End User, 2023 & 2033
Figure 49: Argentina Market Value Proportion Analysis, 2022
Figure 50: Global Vs Argentina Growth Comparison
Figure 51: Argentina Market Share Analysis (%) By Product, 2023 & 2033
Figure 52: Argentina Market Share Analysis (%) By End User, 2023 & 2033
Figure 53: Europe Market Value (US$ Million) Analysis, 2012 to 2022
Figure 54: Europe Market Value (US$ Million) Forecast, 2022 to 2032
Figure 55: Europe Market Value Share, By Product (2023 E)
Figure 56: Europe Market Value Share, By End User (2023 E)
Figure 57: Europe Market Value Share, by Country (2023 E)
Figure 58: Europe Market Attractiveness Analysis By Product, 2023 to 2033
Figure 59: Europe Market Attractiveness Analysis By End User, 2023 to 2033
Figure 60: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 61: UK Market Value Proportion Analysis, 2022
Figure 62: Global Vs. UK Growth Comparison
Figure 63: UK Market Share Analysis (%) By Product, 2023 & 2033
Figure 64: UK Market Share Analysis (%) By End User, 2023 & 2033
Figure 65: Germany Market Value Proportion Analysis, 2022
Figure 66: Global Vs. Germany Growth Comparison
Figure 67: Germany Market Share Analysis (%) By Product, 2023 & 2033
Figure 68: Germany Market Share Analysis (%) By End User, 2023 & 2033
Figure 69: Italy Market Value Proportion Analysis, 2022
Figure 70: Global Vs. Italy Growth Comparison
Figure 71: Italy Market Share Analysis (%) By Product, 2023 & 2033
Figure 72: Italy Market Share Analysis (%) By End User, 2023 & 2033
Figure 73: France Market Value Proportion Analysis, 2022
Figure 74: Global Vs France Growth Comparison
Figure 75: France Market Share Analysis (%) By Product, 2023 & 2033
Figure 76: France Market Share Analysis (%) By End User, 2023 & 2033
Figure 77: Spain Market Value Proportion Analysis, 2022
Figure 78: Global Vs Spain Growth Comparison
Figure 79: Spain Market Share Analysis (%) By Product, 2023 & 2033
Figure 80: Spain Market Share Analysis (%) By End User, 2023 & 2033
Figure 81: Russia Market Value Proportion Analysis, 2022
Figure 82: Global Vs Russia Growth Comparison
Figure 83: Russia Market Share Analysis (%) By Product, 2023 & 2033
Figure 84: Russia Market Share Analysis (%) By End User, 2023 & 2033
Figure 85: BENELUX Market Value Proportion Analysis, 2022
Figure 86: Global Vs BENELUX Growth Comparison
Figure 87: BENELUX Market Share Analysis (%) By Product, 2023 & 2033
Figure 88: BENELUX Market Share Analysis (%) By End User, 2023 & 2033
Figure 89: East Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 90: East Asia Market Value (US$ Million) Forecast, 2022 to 2032
Figure 91: East Asia Market Value Share, By Product (2023 E)
Figure 92: East Asia Market Value Share, By End User (2023 E)
Figure 93: East Asia Market Value Share, by Country (2023 E)
Figure 94: East Asia Market Attractiveness Analysis By Product, 2023 to 2033
Figure 95: East Asia Market Attractiveness Analysis By End User, 2023 to 2033
Figure 96: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 97: China Market Value Proportion Analysis, 2022
Figure 98: Global Vs. China Growth Comparison
Figure 99: China Market Share Analysis (%) By Product, 2023 & 2033
Figure 100: China Market Share Analysis (%) By End User, 2023 & 2033
Figure 101: Japan Market Value Proportion Analysis, 2022
Figure 102: Global Vs. Japan Growth Comparison
Figure 103: Japan Market Share Analysis (%) By Product, 2023 & 2033
Figure 104: Japan Market Share Analysis (%) By End User, 2023 & 2033
Figure 105: South Korea Market Value Proportion Analysis, 2022
Figure 106: Global Vs South Korea Growth Comparison
Figure 107: South Korea Market Share Analysis (%) By Product, 2023 & 2033
Figure 108: South Korea Market Share Analysis (%) By End User, 2023 & 2033
Figure 109: South Asia Market Value (US$ Million) Analysis, 2012 to 2022
Figure 110: South Asia Market Value (US$ Million) Forecast, 2022 to 2032
Figure 111: South Asia Market Value Share, By Product (2023 E)
Figure 112: South Asia Market Value Share, By End User (2023 E)
Figure 113: South Asia Market Value Share, by Country (2023 E)
Figure 114: South Asia Market Attractiveness Analysis By Product, 2023 to 2033
Figure 115: South Asia Market Attractiveness Analysis By End User, 2023 to 2033
Figure 116: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 117: India Market Value Proportion Analysis, 2022
Figure 118: Global Vs. India Growth Comparison
Figure 119: India Market Share Analysis (%) By Product, 2023 & 2033
Figure 120: India Market Share Analysis (%) By End User, 2023 & 2033
Figure 121: Indonesia Market Value Proportion Analysis, 2022
Figure 122: Global Vs. Indonesia Growth Comparison
Figure 123: Indonesia Market Share Analysis (%) By Product, 2023 & 2033
Figure 124: Indonesia Market Share Analysis (%) By End User, 2023 & 2033
Figure 125: Malaysia Market Value Proportion Analysis, 2022
Figure 126: Global Vs. Malaysia Growth Comparison
Figure 127: Malaysia Market Share Analysis (%) By Product, 2023 & 2033
Figure 128: Malaysia Market Share Analysis (%) By End User, 2023 & 2033
Figure 129: Thailand Market Value Proportion Analysis, 2022
Figure 130: Global Vs. Thailand Growth Comparison
Figure 131: Thailand Market Share Analysis (%) By Product, 2023 & 2033
Figure 132: Thailand Market Share Analysis (%) By End User, 2023 & 2033
Figure 133: Oceania Market Value (US$ Million) Analysis, 2012 to 2022
Figure 134: Oceania Market Value (US$ Million) Forecast, 2022 to 2032
Figure 135: Oceania Market Value Share, By Product (2023 E)
Figure 136: Oceania Market Value Share, By End User (2023 E)
Figure 137: Oceania Market Value Share, by Country (2023 E)
Figure 138: Oceania Market Attractiveness Analysis By Product, 2023 to 2033
Figure 139: Oceania Market Attractiveness Analysis By End User, 2023 to 2033
Figure 140: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 141: Australia Market Value Proportion Analysis, 2022
Figure 142: Global Vs. Australia Growth Comparison
Figure 143: Australia Market Share Analysis (%) By Product, 2023 & 2033
Figure 144: Australia Market Share Analysis (%) By End User, 2023 & 2033
Figure 145: New Zealand Market Value Proportion Analysis, 2022
Figure 146: Global Vs New Zealand Growth Comparison
Figure 147: New Zealand Market Share Analysis (%) By Product, 2023 & 2033
Figure 148: New Zealand Market Share Analysis (%) By End User, 2023 & 2033
Figure 149: Middle East & Africa Market Value (US$ Million) Analysis, 2012 to 2022
Figure 150: Middle East & Africa Market Value (US$ Million) Forecast, 2022 to 2032
Figure 151: Middle East & Africa Market Value Share, By Product (2023 E)
Figure 152: Middle East & Africa Market Value Share, By End User (2023 E)
Figure 153: Middle East & Africa Market Value Share, by Country (2023 E)
Figure 154: Middle East & Africa Market Attractiveness Analysis By Product, 2023 to 2033
Figure 155: Middle East & Africa Market Attractiveness Analysis By End User, 2023 to 2033
Figure 156: Middle East & Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 157: GCC Countries Market Value Proportion Analysis, 2022
Figure 158: Global Vs GCC Countries Growth Comparison
Figure 159: GCC Countries Market Share Analysis (%) By Product, 2023 & 2033
Figure 160: GCC Countries Market Share Analysis (%) By End User, 2023 & 2033
Figure 161: Türkiye Market Value Proportion Analysis, 2022
Figure 162: Global Vs. Türkiye Growth Comparison
Figure 163: Türkiye Market Share Analysis (%) By Product, 2023 & 2033
Figure 164: Türkiye Market Share Analysis (%) By End User, 2023 & 2033
Figure 165: South Africa Market Value Proportion Analysis, 2022
Figure 166: Global Vs. South Africa Growth Comparison
Figure 167: South Africa Market Share Analysis (%) By Product, 2023 & 2033
Figure 168: South Africa Market Share Analysis (%) By End User, 2023 & 2033
Figure 169: Northern Africa Market Value Proportion Analysis, 2022
Figure 170: Global Vs Northern Africa Growth Comparison
Figure 171: Northern Africa Market Share Analysis (%) By Product, 2023 & 2033
Figure 172: Northern Africa Market Share Analysis (%) By End User, 2023 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Peripheral Intravenous Catheter Market Size and Share Forecast Outlook 2025 to 2035
Peripheral T-Cell Lymphoma Treatment Market Size and Share Forecast Outlook 2025 to 2035
Peripheral Embolization Device Market Size and Share Forecast Outlook 2025 to 2035
Peripheral Vascular Devices Market Size and Share Forecast Outlook 2025 to 2035
Peripheral Vascular Stent Market Analysis - Size, Trends & Forecast 2025 to 2035
Peripherally Inserted Central Catheter Analysis by Product Type by Indication and by End User through 2035
Peripheral Micro Catheters Market Trends – Industry Forecast 2024-2034
Peripheral Neuritis Treatment Market
Computer Peripherals Market Size and Share Forecast Outlook 2025 to 2035
Continuous Peripheral Nerve Block Catheter Market Growth – Trends & Forecast 2025-2035
The Dual Balloon Angioplasty Catheter Market is segmented by Peripheral, and Coronal from 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA