The global external anti-infective preparations market is projected to grow from USD 5,412.8 million in 2025 to approximately USD 6,728.7 million by 2035, recording an absolute increase of USD 1,315.9 million over the forecast period. This translates into a total growth of 24.3%, with the market forecast to expand at a CAGR of 2.2% between 2025 and 2035. The market size is expected to grow by nearly 1.24X during the same period, supported by the rising prevalence of external infections and increasing demand for localized antimicrobial treatment solutions across diverse healthcare settings.
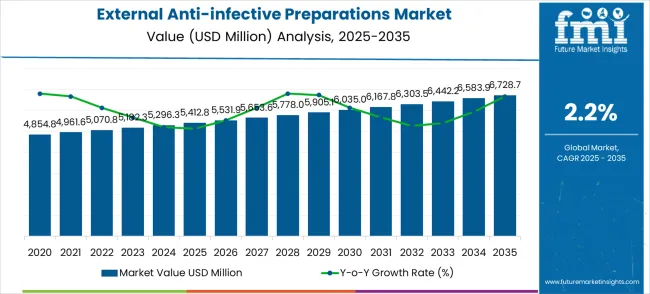
| External Anti-infective Preparations Market | Value |
|---|---|
| Market Value (2025) | USD 5,412.8 million |
| Market Forecast Value (2035) | USD 6,728.7 million |
| Market Forecast CAGR | 2.2% |
The external anti-infective preparations market is closely influenced by five interconnected parent markets that collectively drive its adoption and long-term growth. The largest contributor is the dermatology and skincare industry, which accounts for about 38% share, as topical anti-infectives are widely used for treating bacterial, fungal, and viral skin infections, wound care, and acne management. The surgical and wound management sector contributes around 27%, driven by the application of antiseptics, disinfectants, and antibiotic ointments to reduce infection risks and support post-operative recovery. The hospital and clinical healthcare market holds close to 16% influence, supported by the extensive use of topical anti-infectives in outpatient procedures, emergency care, and burn treatment protocols. The personal care and hygiene sector adds nearly 11%, as antiseptic creams, gels, and sprays are incorporated into everyday healthcare routines to maintain skin integrity and reduce microbial exposure. The veterinary and animal healthcare segment contributes close to 8%, as topical anti-infectives are increasingly applied in treating skin infections, wounds, and preventive care in companion and livestock animals.
Dermatology and wound management form the backbone of the external anti-infective preparations market, while hospital use, personal hygiene, and veterinary applications steadily expand its commercial footprint. This interconnected ecosystem indicates that the market is supported by multiple healthcare and consumer segments, ensuring long-term resilience, steady demand, and consistent year-on-year growth.
Market expansion is being supported by the increasing incidence of external infections worldwide and the corresponding need for effective localized antimicrobial treatments that provide superior therapeutic outcomes with minimized systemic exposure. Modern healthcare systems rely on comprehensive infection management and prevention protocols to ensure optimal patient care, including skin and soft tissue infections, wound care management, and specialized external antimicrobial applications. Even minor external infections can progress to serious complications requiring prompt antimicrobial intervention to maintain optimal patient safety standards and clinical effectiveness.
The growing complexity of antimicrobial resistance challenges and increasing demand for targeted therapeutic approaches are driving demand for external anti-infective preparations from established pharmaceutical manufacturers with appropriate clinical efficacy capabilities and regulatory compliance expertise. Healthcare institutions and medical professionals are increasingly requiring documented antimicrobial effectiveness and safety profiles to maintain treatment quality and infection control standards. Clinical guidelines and infection prevention standards are establishing standardized external infection management procedures that require specialized anti-infective preparations and trained healthcare personnel.
The external anti-infective preparations market is entering a mature development phase driven by increasing infection control awareness, rising demand for localized antimicrobial therapy, and healthcare quality improvement initiatives. As healthcare providers, pharmaceutical companies, and medical institutions across both developed and emerging markets seek treatments that deliver superior antimicrobial efficacy, reduced systemic impact, and comprehensive infection prevention capabilities, external anti-infective preparations are evolving from traditional antimicrobial products to sophisticated therapeutic solutions integrated with advanced delivery mechanisms, combination antimicrobial systems, and evidence-based treatment protocols.
Rising healthcare quality standards and infection prevention focus in Asia-Pacific, Latin America, and Middle East & Africa amplify demand, while manufacturers are advancing innovations in antimicrobial formulation, delivery optimization, and resistance prevention features. Pathways like advanced delivery systems, combination antimicrobial development, and specialized application solutions promise moderate margin enhancement in this established market. Geographic expansion and healthcare infrastructure development will capture volume growth, particularly where medical care standards are improving or infection control programs are expanding. Healthcare quality pressures, antimicrobial stewardship initiatives, and patient safety standards provide structural market support.
The market is segmented by product type, application, and region. By product type, the market is divided into solution, ointment, gel, and other formulations. Based on application, the market is categorized into hospital, retail pharmacy, and other distribution channels. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
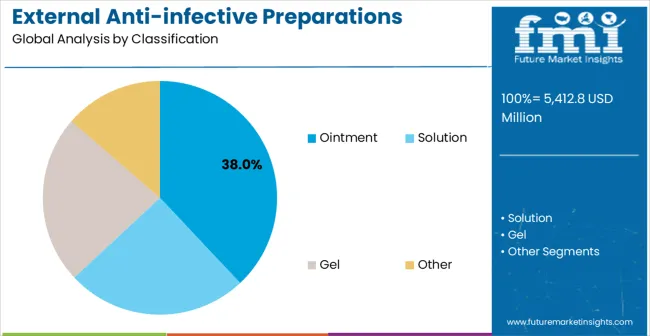
In 2025, the ointment segment is projected to capture around 38% of the total market share, making it the leading product type category. This dominance is largely driven by the widespread preference for ointment-based external anti-infective preparations that deliver antimicrobial release with excellent adherence and moisture barrier properties. The ointment segment is particularly favored for its ability to provide prolonged antimicrobial contact time and enhanced therapeutic penetration while maintaining ease of application and patient comfort. Healthcare professionals, infection control specialists, wound care practitioners, and patients increasingly prefer ointment formulations, as they meet diverse external infection treatment requirements without imposing excessive reapplication frequency or complex usage protocols. The availability of well-established ointment formulations, along with comprehensive clinical evidence and application expertise from leading pharmaceutical manufacturers, further reinforces the segment's market position. This product type benefits from consistent demand across regions, as it is considered a reliable solution for applications requiring antimicrobial delivery and protective barrier function. The combination of antimicrobial efficacy, application convenience, and therapeutic versatility makes ointment formulations a preferred choice, ensuring continued popularity in the external anti-infective preparations market. Advanced formulation technologies and enhanced antimicrobial delivery capabilities continue to drive adoption among healthcare professionals seeking superior infection treatment outcomes.
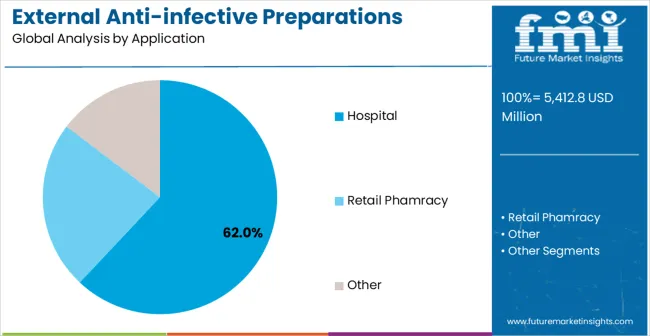
The hospital segment is expected to represent 62% of external anti-infective preparations demand in 2025, highlighting its position as the most significant application sector. This dominance stems from the critical importance of infection prevention and control in healthcare facilities, where effective external antimicrobial management and patient safety protocols are essential to maintaining quality care and preventing healthcare-associated infections. Hospital applications often feature diverse patient populations requiring specialized external infection management throughout extended care periods, demanding reliable and clinically proven anti-infective preparations. External anti-infective preparations are particularly well-suited to hospital applications due to their ability to provide targeted antimicrobial therapy with localized action while supporting comprehensive infection control and prevention protocols. As healthcare facilities expand globally and emphasize improved infection prevention and patient safety standards, the demand for external anti-infective preparations continues to rise. The segment also benefits from heightened focus on healthcare quality initiatives, where medical institutions are increasingly prioritizing evidence-based infection control and prevention strategies as key components of comprehensive patient safety programs. With hospitals investing in infection prevention enhancement and clinical quality improvement, external anti-infective preparations provide an essential solution to maintain high-performance infection control capabilities. The growth of specialized infection control programs, coupled with increased focus on healthcare quality and patient safety, ensures that hospitals will remain the largest and most stable demand driver for external anti-infective preparations in the forecast period. Enhanced antimicrobial formulations and improved safety profiles continue to strengthen the segment's market position.
The External Anti-infective Preparations market is advancing steadily due to increasing healthcare-associated infection concerns and growing recognition of external antimicrobial therapy advantages over systemic treatments in appropriate clinical applications. The market faces challenges including regulatory complexity for antimicrobial product approvals, increasing development costs for innovative formulations, and growing concerns about antimicrobial resistance development with external antimicrobial use. Clinical research efforts and antimicrobial stewardship programs continue to influence product development and market adoption patterns.
The growing development of advanced antimicrobial formulation systems is enabling improved therapeutic effectiveness with enhanced tissue penetration and antimicrobial action characteristics. Enhanced formulation technologies and optimized delivery approaches provide superior antimicrobial outcomes while maintaining safety and tolerability standards required for external applications. These technologies are particularly valuable for healthcare and clinical applications that require reliable antimicrobial performance supporting diverse patient populations with consistent efficacy and safety considerations.
Modern external anti-infective preparation manufacturers are incorporating advanced combination formulations and comprehensive multi-action therapeutic systems that enhance antimicrobial effectiveness while supporting tissue healing and repair processes. Integration of synergistic antimicrobial agents and optimized therapeutic combinations enables superior clinical outcomes and comprehensive external infection management capabilities. Advanced combination features support treatment in diverse clinical environments while meeting various therapeutic requirements and patient specifications, including bacterial infections, fungal conditions, and specialized wound care applications.
The continuous development of evidence-based treatment approaches and clinical optimization principles is improving therapeutic outcomes while maintaining consistent safety profiles throughout treatment protocols. Advanced clinical evidence and intelligent treatment selection provide optimized therapeutic capabilities that support comprehensive patient care activities without compromising antimicrobial effectiveness or patient safety. These evidence-based innovations are particularly valuable for healthcare providers conducting specialized infection management where clinical outcomes and safety considerations are essential for effective treatment and optimal patient care.
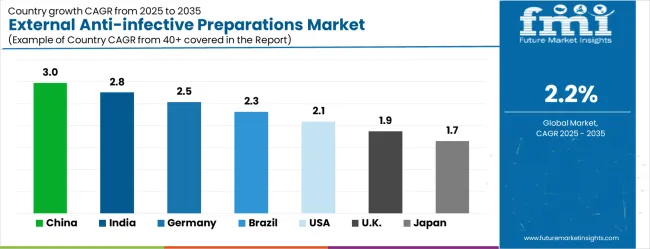
| Country | CAGR (2025-2035) |
|---|---|
| China | 3% |
| India | 2.8% |
| Germany | 2.5% |
| Brazil | 2.3% |
| United States | 2.1% |
| United Kingdom | 1.9% |
| Japan | 1.7% |
The external anti-infective preparations market is growing moderately, with China leading at a 3% CAGR through 2035, driven by expanding healthcare infrastructure and increasing awareness of external infection treatment options. India follows at 2.8%, supported by rising healthcare access and growing pharmaceutical market development. Germany grows steadily at 2.5%, integrating advanced external antimicrobial therapies into its established healthcare system. Brazil records 2.3%, emphasizing healthcare modernization and pharmaceutical access enhancement. The United States shows solid growth at 2.1%, focusing on clinical innovation and infection control excellence. The United Kingdom demonstrates steady progress at 1.9%, maintaining established infection prevention standards. Japan records 1.7% growth, concentrating on clinical optimization and therapeutic quality enhancement.
The report covers an in-depth analysis of 40+ countries, top-performing countries are highlighted below.
Revenue from external anti-infective preparations in China is projected to exhibit the highest growth rate with a CAGR of 3% through 2035, driven by rapid expansion of healthcare infrastructure and increasing awareness of external antimicrobial treatment benefits for infection management. The country's growing focus on healthcare quality improvement and expanding medical service delivery is creating significant demand for effective external anti-infective treatment solutions. Major pharmaceutical companies and healthcare distributors are establishing comprehensive supply networks to support the increasing requirements of hospitals and healthcare facilities across urban and rural medical applications. Government healthcare development initiatives are supporting establishment of modern infection control capabilities and advanced pharmaceutical access systems, driving demand for sophisticated external anti-infective preparation portfolios throughout major medical centers and community healthcare facilities. Healthcare modernization programs are facilitating adoption of evidence-based external antimicrobial therapies that enhance infection control effectiveness and patient care standards across healthcare networks. The Chinese government's commitment to universal healthcare quality and improved infection prevention is accelerating investments in pharmaceutical procurement including external anti-infective preparations. Urban healthcare development projects and community health initiatives are incorporating comprehensive infection control protocols that require reliable external antimicrobial therapeutic options. Commercial healthcare expansion and pharmaceutical access programs are creating additional demand for professional-grade external anti-infective preparations that meet international quality standards and clinical effectiveness requirements.
Revenue from external anti-infective preparations in India is expanding at a CAGR of 2.8%, supported by increasing healthcare access development and growing awareness of external antimicrobial treatment approaches. The country's expanding healthcare infrastructure and rising medical service quality standards are driving demand for reliable external anti-infective therapeutic solutions. Healthcare providers and pharmaceutical distributors are gradually implementing comprehensive external anti-infective preparation portfolios to maintain infection control standards and clinical effectiveness. Healthcare sector growth and infrastructure development are creating opportunities for pharmaceutical suppliers that can support diverse treatment requirements and clinical applications. Professional medical training and pharmaceutical education programs are building clinical expertise among healthcare personnel, enabling effective utilization of external anti-infective preparations that meet infection control standards and patient care requirements across medical regions. India's rapid healthcare development and increasing pharmaceutical market expansion are intensifying the need for effective external infection management and antimicrobial care capabilities. National healthcare development programs and medical facility expansion projects are establishing comprehensive infection control systems that require advanced pharmaceutical therapeutic options. State government initiatives focusing on healthcare improvement and pharmaceutical access are driving procurement of modern external anti-infective preparations for hospitals and healthcare facilities.
Demand for external anti-infective preparations in Germany is projected to grow at a CAGR of 2.5%, supported by the country's emphasis on clinical excellence and advanced infection control standard adoption. German healthcare providers and medical institutions are implementing sophisticated external anti-infective treatment protocols that meet stringent clinical requirements and safety specifications. The market is characterized by a focus on antimicrobial efficacy, patient safety, and compliance with comprehensive infection control standards. Healthcare system investments are prioritizing evidence-based therapeutic options that demonstrate superior clinical outcomes and safety profiles while meeting German medical and regulatory standards. Professional medical education programs are ensuring comprehensive clinical expertise among healthcare personnel, enabling specialized therapeutic capabilities that support diverse infection control applications and treatment requirements across healthcare networks. Germany's commitment to maintaining the highest medical care and infection control standards in Europe drives continuous investment in advanced antimicrobial treatments. The country's comprehensive healthcare infrastructure requires sophisticated therapeutic options that meet strict clinical effectiveness and safety control standards. Integration with digital healthcare systems and evidence-based treatment protocols is creating demand for advanced external anti-infective preparations with documented clinical capabilities.
Revenue from external anti-infective preparations in Brazil is growing at a CAGR of 2.3%, driven by increasing healthcare system development and growing recognition of external antimicrobial therapy advantages for infection management. The country's expanding healthcare service system is gradually integrating comprehensive external anti-infective treatment options to enhance clinical capabilities and infection control effectiveness. Healthcare providers and medical facilities are investing in pharmaceutical portfolios to address evolving infection management requirements and patient care standards. Healthcare modernization is facilitating adoption of evidence-based therapeutic options that support comprehensive infection control capabilities across medical service regions. Professional medical development programs are enhancing clinical capabilities among healthcare personnel, enabling effective external anti-infective preparation utilization that meets evolving patient care standards and infection control requirements throughout healthcare networks. Brazil's expanding healthcare sector and growing pharmaceutical market are driving demand for reliable clinical infection management options. Federal and state government initiatives focused on improving healthcare capabilities and pharmaceutical access are supporting procurement of modern external anti-infective preparations. Healthcare system development and increasing medical service efficiency in major metropolitan areas require enhanced infection control capabilities that can support effective patient care operations.
Demand for external anti-infective preparations in the USA is expanding at a CAGR of 2.1%, driven by established healthcare systems and growing emphasis on infection control innovation enhancement. Large healthcare organizations and medical institutions are implementing comprehensive external anti-infective capabilities to serve diverse patient treatment requirements while meeting clinical quality standards. The market benefits from established pharmaceutical distribution networks and professional medical training programs that support various clinical applications. Healthcare industry leadership is enabling standardized therapeutic utilization across multiple medical specialties, providing consistent clinical effectiveness and comprehensive infection control coverage throughout regional markets. Professional medical development and certification programs are building specialized clinical expertise among healthcare personnel, enabling effective external anti-infective preparation utilization that supports evolving patient care requirements across medical areas. The USA market is characterized by continuous therapeutic advancement and integration with modern evidence-based medicine systems. Federal healthcare programs are driving utilization of clinically proven therapeutic options that meet stringent effectiveness and safety standards. Healthcare organizations and medical groups are modernizing their infection control capabilities through adoption of advanced external anti-infective therapies.
Demand for external anti-infective preparations in the UK is projected to grow at a CAGR of 1.9%, supported by established healthcare sectors and growing emphasis on clinical infection control capabilities. British healthcare services and medical institutions are implementing external anti-infective treatment protocols that meet healthcare system performance standards and clinical requirements. The market benefits from an established medical care infrastructure and comprehensive training programs for healthcare professionals. Healthcare quality investments are prioritizing evidence-based therapeutic options that support diverse clinical applications while maintaining established effectiveness and safety standards. Professional medical development programs are building clinical expertise among healthcare personnel, enabling specialized external anti-infective preparation utilization capabilities that meet evolving treatment requirements and quality standards throughout regional healthcare networks. The United Kingdom's comprehensive healthcare strategy and commitment to clinical excellence drive continued investment in proven therapeutic options. Medical services across England, Wales, Scotland, and Northern Ireland are standardizing their infection control protocols to ensure consistent clinical care capabilities. Integration with national healthcare monitoring systems and coordination with clinical quality programs requires reliable external anti-infective preparations.
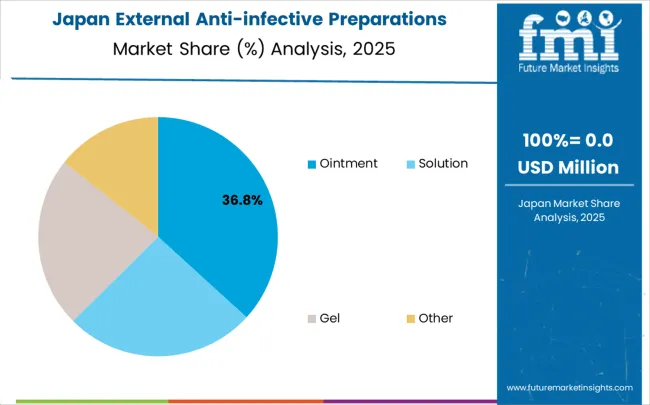
Revenue from external anti-infective preparations in Japan is growing at a CAGR of 1.7%, driven by the country's focus on clinical refinement and therapeutic quality enhancement applications. Japanese healthcare providers and medical institutions are implementing advanced external anti-infective treatment systems that demonstrate superior clinical reliability and infection control effectiveness. The market is characterized by emphasis on therapeutic excellence, clinical quality assurance, and integration with established medical care workflows. Healthcare industry investments are prioritizing refined therapeutic solutions that combine advanced clinical evidence with superior safety profiles while maintaining Japanese medical and quality standards. Professional medical development programs ensure comprehensive clinical expertise among healthcare personnel, enabling specialized therapeutic capabilities that support diverse medical applications and infection control requirements throughout metropolitan healthcare networks. Japan's commitment to maintaining the highest standards of medical care and clinical quality drives continuous refinement in therapeutic approaches. The integration of external anti-infective preparations with comprehensive medical care systems and evidence-based treatment protocols requires sophisticated clinical capabilities. Healthcare organizations across Japan's medical regions are optimizing their infection control approaches through utilization of clinically refined therapeutic options that meet strict effectiveness and safety standards.
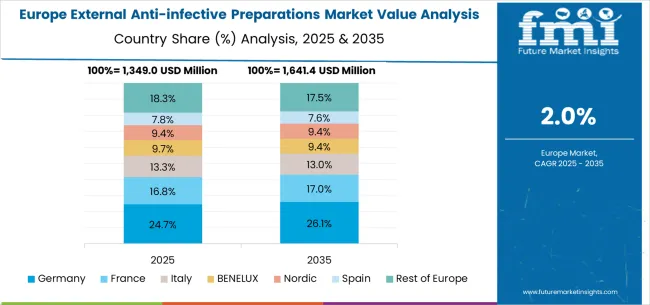
The external anti-infective preparations market in Europe is anticipated to expand from USD 1,286.4 million in 2025 to approximately USD 1,502.8 million by 2035, reflecting a CAGR of 1.6% during the forecast period. Germany is set to reinforce its leading position, commanding a 29.2% share of the European market in 2025, supported by its established healthcare infrastructure, high standards for clinical care, and continuous investment in evidence-based antimicrobial therapeutic options. The United Kingdom follows with a market share of 17.8%, benefiting from standardized medical care procedures, professional healthcare training programs, and a robust focus on infection control quality modernization. France is projected to capture 15.9% of the regional market, fueled by steady integration of modern therapeutic options and enhancements in medical care operations. Italy and Spain together represent 21.6% of total demand, as both countries advance their healthcare modernization efforts and invest in comprehensive therapeutic solutions for medical institutions. The Rest of Europe, comprising Nordic countries, BENELUX, and Eastern European markets, accounts for 15.5%, driven by regulatory convergence, healthcare infrastructure upgrading, and broader adoption of evidence-based external anti-infective preparations to support evolving medical care requirements and infection control programs.
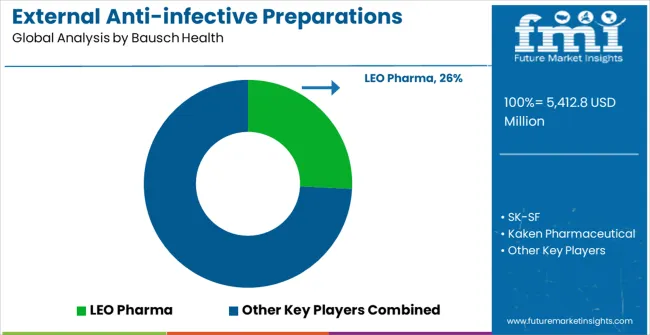
The External Anti-infective Preparations market is defined by competition among established pharmaceutical companies, infection control specialists, and generic pharmaceutical manufacturers. Companies are investing in clinical research and development, formulation innovation, regulatory compliance, and comprehensive market access capabilities to deliver effective, safe, and accessible external antimicrobial treatment solutions. Strategic partnerships, clinical validation, and geographic expansion are central to strengthening product portfolios and market presence.
Bausch Health offers comprehensive external anti-infective solutions with established clinical expertise and professional-grade antimicrobial capabilities. LEO Pharma provides specialized dermatological and infection control treatments with a focus on clinical efficacy and patient care quality. SK-SF delivers advanced pharmaceutical solutions with emphasis on therapeutic innovation and healthcare market access. Kaken Pharmaceutical specializes in external antimicrobial therapeutics with advanced formulation development integration.
Haleon offers consumer healthcare and pharmaceutical products with comprehensive therapeutic support capabilities. Bayer delivers established pharmaceutical solutions with advanced anti-infective technologies. Johnson & Johnson provides comprehensive healthcare solutions with a focus on clinical excellence and infection control. STADA Arzneimittel, Galderma, and multiple regional and international manufacturers offer specialized pharmaceutical expertise, therapeutic reliability, and comprehensive product development across global and regional market segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 5,412.8 million |
| Product Type | Solution, Ointment, Gel |
| Application | Hospital, Retail Pharmacy |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | Bausch Health, LEO Pharma, SK-SF, Kaken Pharmaceutical, Haleon, Bayer, Johnson & Johnson, STADA Arzneimittel, Galderma, Zhejiang Funo Pharmaceuticals, Jiangsu Fubang Pharmaceuticals |
| Additional Attributes | Dollar sales by product type and application segment, regional demand trends across major markets, competitive landscape with established pharmaceutical manufacturers and emerging therapeutic providers, healthcare provider preferences for different formulation types and treatment approaches, integration with medical care systems and infection control protocols, innovations in antimicrobial delivery and therapeutic effectiveness technologies, and adoption of evidence-based treatment features with enhanced clinical capabilities for improved patient care workflows. |
The global external anti-infective preparations market is estimated to be valued at USD 5,412.8 million in 2025.
The market size for the external anti-infective preparations market is projected to reach USD 6,728.7 million by 2035.
The external anti-infective preparations market is expected to grow at a 2.2% CAGR between 2025 and 2035.
The key product types in external anti-infective preparations market are ointment, solution, gel and other.
In terms of application, hospital segment to command 62.0% share in the external anti-infective preparations market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
External Anthelmintic for Dogs Market Size and Share Forecast Outlook 2025 to 2035
External Storage Market Size and Share Forecast Outlook 2025 to 2035
External Fixator Market Size and Share Forecast Outlook 2025 to 2035
External Blinds Market Size and Share Forecast Outlook 2025 to 2035
External Pacemakers Market Size and Share Forecast Outlook 2025 to 2035
External Defibrillators Market Size and Share Forecast Outlook 2025 to 2035
External Gear Pump Market Size, Growth, and Forecast 2025 to 2035
External Combustion Engine Market Growth & Demand 2025 to 2035
Animal External Fixation Market Size and Share Forecast Outlook 2025 to 2035
Automated External Defibrillator Market Analysis – Size, Share, and Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA