The healthcare regulatory affairs outsourcing market is expanding steadily due to increasing complexity in global compliance requirements and the growing need for specialized expertise. Market growth is being driven by rising demand from pharmaceutical, biotechnology, and medical device companies seeking to streamline approval timelines and reduce operational costs. The current environment reflects heightened regulatory scrutiny and evolving regional standards, prompting organizations to rely on outsourcing partners with strong domain knowledge and global reach.
Technological advancements in digital documentation, data analytics, and automation are further improving efficiency and accuracy in submission processes. The future outlook remains positive as life sciences companies continue to expand into emerging markets, requiring localized regulatory support and adaptive compliance strategies.
The growth rationale lies in cost optimization, faster market access, and strategic focus on core research and development functions As regulatory expectations intensify globally, outsourcing partnerships are expected to remain integral to maintaining operational agility, compliance assurance, and sustainable business continuity.

| Metric | Value |
|---|---|
| Healthcare Regulatory Affairs Outsourcing Market Estimated Value in (2025 E) | USD 2.3 billion |
| Healthcare Regulatory Affairs Outsourcing Market Forecast Value in (2035 F) | USD 4.3 billion |
| Forecast CAGR (2025 to 2035) | 6.7% |
The market is segmented by Services and End User and region. By Services, the market is divided into Regulatory Writing and Publishing, Regulatory Submissions, Clinical Trial Applications, Product Registrations, Regulatory Consulting, and Legal Representation. In terms of End User, the market is classified into Pharmaceutical Companies, Biotechnology Companies, Medical Devices Manufacturer, and Food And Beverage Companies. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.

The regulatory writing and publishing segment, accounting for 27.4% of the services category, has emerged as the leading service area owing to its critical role in preparing, compiling, and submitting documentation in line with stringent international standards. Growth has been supported by the increasing number of product approvals, clinical trials, and post-market surveillance activities that require comprehensive documentation.
Outsourcing of these functions has been preferred to ensure accuracy, regulatory alignment, and time efficiency. The segment benefits from the adoption of advanced software tools that facilitate automated formatting and version control, reducing errors and enhancing submission quality.
Regulatory writing and publishing services are increasingly being sought by both established and emerging healthcare firms aiming to manage submission volumes effectively Continued expansion of regulatory frameworks across regions is expected to sustain the segment’s prominence as companies prioritize compliance efficiency and consistency in documentation standards.

The pharmaceutical companies segment, holding 42.8% of the end-user category, has maintained dominance due to the extensive regulatory demands associated with drug development, approval, and commercialization. Outsourcing of regulatory affairs allows pharmaceutical firms to accelerate submission processes, manage complex global compliance requirements, and optimize internal resource utilization.
The segment’s leadership is being reinforced by growing R&D pipelines, increasing clinical trial activity, and rising demand for faster market entry across therapeutic categories. Partnerships with specialized service providers enable pharmaceutical companies to access regional expertise, enhance document standardization, and ensure adherence to evolving guidelines from agencies such as the FDA and EMA.
The trend toward digital transformation and regulatory information management systems has further strengthened collaboration between pharma companies and outsourcing partners Sustained market share is anticipated as pharmaceutical firms continue leveraging outsourcing to balance cost efficiency, compliance accuracy, and strategic agility in a highly regulated global environment.
Market players are offering healthcare firms with functional outsourcing to enhance efficiency. These outsourcing companies offer the healthcare forms to contract multiple monitors (CRAs), coordinators, senior monitors, and project managers for a particular period of time. This would help them to support the internal resources without the expansion of their own clinical team.
The outsourcing services have dedicated clinical experts working under the project management of the clients. They mostly offer FTE based budgets, with predictable and fixed pricing.
Healthcare companies will have a single point of contact with these outsourcing firms. All the staffing requirements, from changing needs to operational and financial management of the projects, will be taken care of.
The global demand for the healthcare regulatory affairs outsourcing market was estimated to reach a valuation of USD 1,123.80 million in 2020, according to a report from Future Market Insights. From 2020 to 2025, the healthcare regulatory affairs outsourcing market witnessed significant growth, registering a CAGR of 12.10%.
| Historical CAGR from 2020 to 2025 | 12.10% |
|---|---|
| Forecast CAGR from 2025 to 2035 | 6.70% |
The growth of biotechnology and pharmaceutical companies is a major factor driving the healthcare regulatory affairs outsourcing market as they require effective regulatory plans and submissions.
Companies are spending more and more on outsourcing partners for specific regulatory knowledge as they struggle to bring new goods to market. This helps them navigate complex regulatory frameworks, gain a competitive edge, and ensure compliance and timely market entrance
Cost Savings, Increased Efficiency and Regulatory Compliance to Push Companies toward these Services
Companies can avoid the overhead expenses associated with having an in-house regulatory affairs staff. With outsourcing, companies may pay for just the work completed, giving them flexibility.
Efficiency gains are possible through outsourcing. There are various kinds of core and non-core activities within a healthcare company. By outsourcing the non-core activities, companies can devote more time towards the core activities and enhance the efficiency.
With the evolving regulatory framework of various bodies, the healthcare companies find it quite difficult to abide by it all at the same time. They prefer to outsource such responsibilities to a third company that holds better knowledge of all these.
High Initials Costs and Technological Challenges to Restrict Market Growth
Healthcare regulatory affairs outsourcing services have a lot of advantages for the healthcare companies. But there are some serious complications with data too. By outsourcing services, healthcare companies will face a loss of control over their own critical processes.
Companies have to provide the outsourcing forms with their complete set of data, which can lead to patient data leak and vulnerabilities. A lack of direct monitoring and control over outsourced medical billing and collections could make patient data vulnerable. Factors such as these can hinder the global market growth eventually.
This section focuses on providing detailed analysis of two particular market segments for the healthcare regulatory affairs outsourcing, the dominant service type and the significant end user. The two main segments discussed below are the regulatory writing and publishing service and mid-size pharmaceutical companies.
| Services | Regulatory Writing and Publishing |
|---|---|
| Market Share in 2025 | 33.60% |
In 2025, the regulatory writing and publishing service was estimated to gain a market share of 33.60%. This is because there is a growing need for thorough documentation and submission assistance.
Companies are looking for qualified experts to guarantee correct and timely submissions due to changing legislation and tighter compliance standards. Companies in the healthcare sector can improve productivity, simplify procedures, and successfully comply with regulations by outsourcing these jobs.
| End User | Mid-Sized Pharmaceutical Companies |
|---|---|
| Market Share in 2025 | 29.20% |
In 2025, the mid-size pharmaceutical companies were estimated to be the dominant end user for the healthcare regulatory affairs outsourcing market. They may accelerate product development schedules, handle complicated compliance needs with efficiency, and concentrate resources on key capabilities by outsourcing regulatory affairs.
The ability to obtain expertise catered to their particular requirements is one notable benefit of outsourcing regulatory affairs for mid-sized pharmaceutical companies. This enables them to successfully negotiate complicated regulatory landscapes while concentrating on the core operations. They may now more successfully compete in the market while still adhering to legal requirements due to this.
This section will go into detail on the healthcare regulatory affairs outsourcing markets in a few key countries, including the United States, the United Kingdom, Spain, Thailand and Indonesia. This part will focus on the key factors that are driving up demand in these countries for the healthcare regulatory affairs outsourcing.
| Countries | CAGR from 2025 to 2035 |
|---|---|
| The United States | 3.40% |
| The United Kingdom | 3.80% |
| Spain | 4.40% |
| Thailand | 5.30% |
| Indonesia | 4.50% |

The United States healthcare regulatory affairs outsourcing ecosystem is anticipated to gain a CAGR of 3.40% through 2035. Continuous innovation in healthcare technology necessitates faster regulatory processes in order to bring novel products to market as quickly as possible.
Outsourcing regulatory affairs enables businesses to use professional expertise and resources to speed approvals, assuring rapid market access while conforming to regulatory requirements.
The healthcare regulatory affairs outsourcing market in the United Kingdom is expected to expand with a 3.80% CAGR through 2035. Brexit, the United Kingdom's exit from the European Union, still causes regulations in the healthcare industry to be out of compliance with EU standards.
Companies who operate in both the United Kingdom's and the European Union's markets face difficulties as a result of this possible regulatory divergence since they have to deal with several regulatory regimes.
After Brexit, specialized knowledge becomes necessary to comprehend and abide by the changing British rules. Companies may conveniently acquire this knowledge by outsourcing regulatory affairs, which guarantees that they can maintain compliance and adjust to new needs while concentrating on their core business operations.
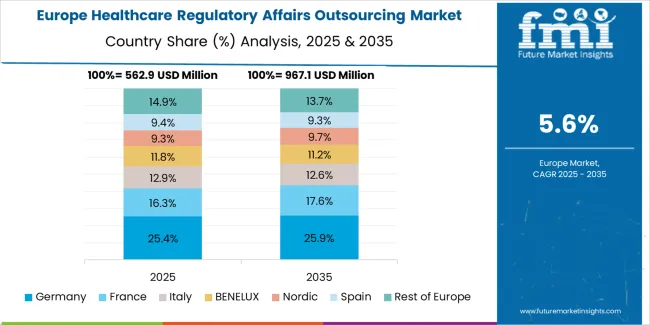
The healthcare regulatory affairs outsourcing ecosystem in Spain is anticipated to develop with a 4.40% CAGR from 2025 to 2035.Spain's distinct cultural and linguistic characteristics necessitate specialized expertise for most effective regulatory compliance.Regulatory affairs might be outsourced to companies with local knowledge to enable more efficient understanding and communication of regulations.
The healthcare regulatory affairs outsourcing industry in Thailand is anticipated to reach a 5.30% CAGR from 2025 to 2035. The healthcare industry in Thailand is at present aimed at aligned its regulations with that of the international norms and standards.
This step requires immense expertise and knowledge, pushing them towards outsourcing agencies. Regulatory affairs outsourcing provides access to expert knowledge necessary for effectively navigating these dynamic regulatory regimes.
The healthcare regulatory affairs outsourcing ecosystem in Indonesia is likely to evolve with a 4.50% CAGR during the forecast period.
Indonesia has got certain strict regulatory bodies for various medical devices and pharmaceutical drugs. These regulations are stated by bodies like the National Agency of Drug and Food Control (NADFC) functioning under the Indonesian Ministry of Health (MoH).
In order to comply with the changing regulations from these bodies, companies require investments to overcome the hurdles. Market players in Indonesia, thus, take help from various healthcare companies to get large scale investments to meet regulatory compliance.

Market players are actively offering specialized regulatory assistance to biotechnology, pharmaceutical, and medical device companies within the healthcare regulatory affairs outsourcing market.
They provide proficiency in navigating through intricate regulatory structures, guaranteeing adherence to legal statutes and guidelines, and hastening the approvals for the research and studies.
Companies also provide a variety of services, such as pharmacovigilance, submission preparation, regulatory strategy development, and compliance monitoring. They want to reduce the regulatory restrictions consumers face in order to promote healthcare facilities more efficiently and in conformity with quality standards.
This suggests that the market players are simply aimed at easing down the various restrictions that organizations face due to legal restrictions. The key players in this market include:
Key Development by Market Players
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 6.7% from 2025 to 2035 |
| Market value in 2025 | USD 2.3 billion |
| Market value in 2035 | USD 4.3 billion |
| Base Year for Estimation | 2025 |
| Historical Data | 2020 to 2025 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | USD million for value |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Services, End User, Region |
| Regions Covered | North America; Latin America; Western Europe; Eastern Europe; South Asia and Pacific; East Asia; The Middle East & Africa |
| Countries Profiled |
The United States, Canada, Brazil, Mexico, Germany, The United Kingdom, France, Spain, Italy, Poland, Russia, Czech Republic, Romania, India, Bangladesh, Australia, New Zealand, China, Japan, South Korea, GCC Countries, South Africa, Israel |
| Key Companies Profiled | Accell Clinical Research LLC; Charles River Laboratories; Syneos Health; Laboratory Corporation of America Holdings; ICON PLC.; IQVIA; Medpace, Inc.; PAREXEL International Corporation; Thermo Fisher Scientific Inc. (PPD); Promedica International; WuXi App Tec |
| Customisation Scope | Available on Request |
The global healthcare regulatory affairs outsourcing market is estimated to be valued at USD 2.3 billion in 2025.
The market size for the healthcare regulatory affairs outsourcing market is projected to reach USD 4.3 billion by 2035.
The healthcare regulatory affairs outsourcing market is expected to grow at a 6.7% CAGR between 2025 and 2035.
The key product types in healthcare regulatory affairs outsourcing market are regulatory writing and publishing, regulatory submissions, clinical trial applications, product registrations, regulatory consulting and legal representation.
In terms of end user, pharmaceutical companies segment to command 42.8% share in the healthcare regulatory affairs outsourcing market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Healthcare IT Outsourcing Market Growth - Analysis & Forecast 2025 to 2035
Competitive Overview of Biologics Regulatory Affairs Market Share
Outsourcing Civil Engineering Services Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Air Purifier Market Size and Share Forecast Outlook 2025 to 2035
Healthcare and Laboratory Label Industry Analysis in the United States Size and Share Forecast Outlook 2025 to 2035
Healthcare Flooring Market Size and Share Forecast Outlook 2025 to 2035
Healthcare AI Computer Vision Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Business Intelligence Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Master Data Management Market Size and Share Forecast Outlook 2025 to 2035
Healthcare and Laboratory Label Industry Analysis in Japan Size and Share Forecast Outlook 2025 to 2035
Healthcare and Laboratory Label Industry Analysis in Western Europe Size and Share Forecast Outlook 2025 to 2035
Healthcare Contact Center Solution Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Semiconductor Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Cold Chain Logistics Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Mobile Computers Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Cloud Infrastructure Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Companion Robots Market Size and Share Forecast Outlook 2025 to 2035
Regulatory Information Management Market Size and Forecast Outlook 2025 to 2035
Healthcare Analytical Testing Services Market Size and Share Forecast Outlook 2025 to 2035
Healthcare Analytics Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA