The human transferrin detection kit industry stands at the threshold of a decade-long expansion trajectory that promises to reshape clinical diagnostics technology and protein biomarker detection solutions. The market's journey from USD 557.9 million in 2025 to USD 962 million by 2035 represents substantial growth, demonstrating the accelerating adoption of advanced immunoassay platforms and quantitative detection technology across hospital laboratories, research facilities, and clinical diagnostic sectors.
The first half of the decade (2025-2030) will witness the market climbing from USD 557.9 million to approximately USD 732.7 million, adding USD 174.8 million in value, which constitutes 43% of the total forecast growth period. This phase will be characterized by the rapid adoption of automated immunoturbidimetric systems, driven by increasing demand for iron metabolism disorder diagnosis and the growing need for high-throughput clinical testing solutions worldwide. Advanced automated analyzers and standardized detection protocols will become standard expectations rather than premium options.
The latter half (2030-2035) will witness sustained growth from USD 732.7 million to USD 962 million, representing an addition of USD 229.4 million or 57% of the decade's expansion. This period will be defined by mass market penetration of point-of-care testing technologies, integration with comprehensive laboratory information management platforms, and seamless compatibility with existing clinical laboratory infrastructure. The market trajectory signals fundamental shifts in how diagnostic facilities approach protein biomarker detection and iron status assessment, with participants positioned to benefit from sustained demand across multiple assay formats and application segments.
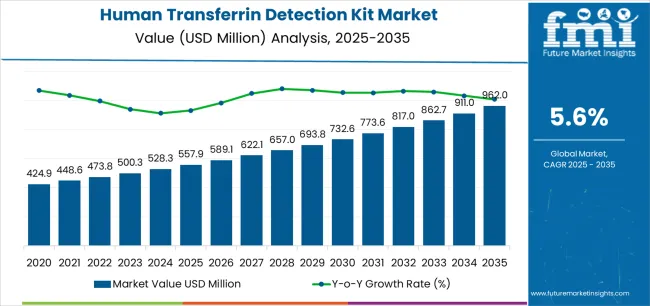
The human transferrin detection kit market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its diagnostic automation phase, expanding from USD 557.9 million to USD 732.7 million with steady annual increments averaging 5.6% growth. This period showcases the transition from manual ELISA protocols to automated immunoturbidimetric systems with enhanced throughput capabilities and integrated quality control becoming mainstream features.
The 2025-2030 phase adds USD 174.8 million to market value, representing 43% of total decade expansion. Market maturation factors include standardization of clinical reference ranges, declining cost per test for automated platforms, and increasing laboratory adoption of high-throughput systems reaching 92-95% analytical accuracy in transferrin quantification. Competitive landscape evolution during this period features established diagnostic companies like Thermo Fisher Scientific and Bio-Techne expanding their immunoassay portfolios while specialty manufacturers focus on advanced detection technology development and enhanced analytical sensitivity capabilities.
From 2030 to 2035, market dynamics shift toward point-of-care integration and global diagnostic standardization, with growth continuing from USD 732.7 million to USD 962 million, adding USD 229.4 million or 57% of total expansion. This phase transition centers on rapid testing platforms, integration with digital laboratory networks, and deployment across diverse clinical scenarios, becoming standard rather than specialized diagnostic applications. The competitive environment matures with focus shifting from basic detection capability to comprehensive diagnostic optimization systems and integration with electronic health record platforms.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 557.9 million |
| Market Forecast (2035) | USD 962 million |
| Growth Rate | 5.6% CAGR |
| Leading Assay Type | Immunoturbidimetric Assay Kit |
| Primary Application | Hospital Segment |
The market demonstrates strong fundamentals with immunoturbidimetric assay kits capturing a dominant share through advanced automation compatibility and rapid turnaround capabilities. Hospital applications drive primary demand, supported by increasing iron deficiency anemia diagnosis requirements and clinical laboratory modernization needs. Geographic expansion remains concentrated in developed markets with established diagnostic infrastructure, while emerging economies show accelerating adoption rates driven by healthcare system development and rising diagnostic standards.
Market expansion rests on three fundamental shifts driving adoption across clinical diagnostic and research sectors. First, iron metabolism disorder prevalence creates compelling diagnostic demand through human transferrin detection kits that provide accurate iron status assessment without invasive procedures, enabling clinicians to meet diagnostic standards while maintaining patient care quality and reducing diagnostic uncertainty. Second, chronic disease monitoring accelerates as healthcare facilities worldwide seek biomarker testing systems that complement routine blood analysis, enabling precise nutritional assessment and therapeutic monitoring that align with clinical protocols and treatment guidelines.
Third, research application expansion drives adoption from pharmaceutical companies and academic institutions requiring standardized protein quantification that enhances experimental reliability while maintaining long-term data consistency during biomarker studies. However, growth faces headwinds from alternative biomarker challenges that vary across diagnostic suppliers regarding ferritin and iron saturation testing, which may limit adoption in cost-sensitive healthcare markets. Technical limitations also persist regarding sample interference issues and matrix effect variations that may reduce accuracy in lipemic or hemolyzed specimens, which affect diagnostic reliability and clinical decision-making.
The human transferrin detection kit market represents a specialized yet essential clinical diagnostics opportunity driven by expanding disease burden awareness, laboratory automation advancement, and the need for superior analytical performance in iron metabolism assessment. As clinical laboratories worldwide seek to achieve 92-95% analytical accuracy, reduce turnaround times, and integrate advanced detection systems with digital laboratory platforms, transferrin detection kits are evolving from basic diagnostic tools to sophisticated biomarker quantification solutions that ensure diagnostic quality and clinical utility.
The convergence of chronic disease prevalence growth, laboratory automation advancement, and analytical technology development creates sustained demand drivers across multiple healthcare segments. The market's growth trajectory from USD 557.9 million in 2025 to USD 962 million by 2035 at a 5.6% CAGR reflects fundamental shifts in clinical laboratory requirements and diagnostic optimization.
Geographic expansion opportunities are particularly pronounced in Asia-Pacific markets, where China (7.6% CAGR) and India (7.0% CAGR) lead through aggressive healthcare infrastructure development and diagnostic laboratory expansion. The dominance of immunoturbidimetric assay systems (58.0% market share) and hospital applications (68.0% share) provides clear strategic focus areas, while emerging point-of-care technology adoption and research application services open new revenue streams across diverse diagnostic markets.
Strengthening the dominant immunoturbidimetric assay segment (58.0% market share) through enhanced analytical sensitivity, superior throughput characteristics, automated analyzer compatibility, and seamless integration with modern laboratory infrastructure. This pathway focuses on optimizing antibody specificity, improving precision performance, extending calibration stability to 30-45 days, and developing specialized protocols for diverse specimen types. Market leadership consolidation through advanced immunochemistry, comprehensive quality assurance, and analyzer integration enables premium positioning while defending competitive advantages against ELISA alternatives. Expected revenue pool: USD 95-125 million
Strategic expansion within ELISA kit technology (32.0% market share) through high-sensitivity detection, flexible sample volume requirements, and comprehensive protocol optimization for research laboratories. This pathway addresses experimental research requirements, biomarker discovery applications, and specialized detection capabilities with advanced colorimetric technology for demanding analytical standards. Premium positioning reflects research-grade quality, protocol flexibility, and comprehensive technical documentation while enabling access to pharmaceutical research programs and academic laboratory networks. Expected revenue pool: USD 52-68 million
Expansion within the dominant hospital segment (68.0% market share) through specialized clinical chemistry formulations, laboratory automation integration programs, and comprehensive technical support for hospital laboratories. This pathway encompasses high-throughput testing systems, quality control integration, cost-effective solutions, and compatibility with diverse analytical platforms. Premium positioning reflects superior analytical reliability, consistent clinical performance, and comprehensive laboratory support that enables modern diagnostic operations while facilitating integration with laboratory information systems and quality management platforms. Expected revenue pool: USD 112-145 million
Development within laboratory research applications (32.0% share) addressing experimental protocol requirements, enhanced detection sensitivity, and specialized assay customization for academic research. This pathway encompasses batch consistency optimization, long-term stability verification, flexible plate formats, and advanced standard curve generation for precision studies. Technology differentiation through proprietary antibody development, application-specific protocols, and comprehensive validation data enables premium pricing while expanding addressable market opportunities across pharmaceutical and biotechnology research segments. Expected revenue pool: USD 52-68 million
Rapid healthcare infrastructure development across China (7.6% CAGR) and India (7.0% CAGR) creates substantial expansion opportunities through local manufacturing capabilities, hospital laboratory partnerships, and comprehensive distribution development. Growing disease burden awareness, diagnostic facility modernization, and healthcare access initiatives drive sustained demand for clinical diagnostic systems. Localization strategies reduce import costs, enable faster regulatory approval, and position companies advantageously for hospital procurement programs while accessing growing domestic markets requiring standardized diagnostic solutions. Expected revenue pool: USD 78-102 million
Development of point-of-care testing platforms (10.0% share) through rapid turnaround protocols, simplified operation procedures, and comprehensive decentralized testing optimization for emergency departments. This pathway encompasses lateral flow technology, portable analyzers, minimal sample requirements, and extensive result interpretation support for non-laboratory healthcare settings. Premium positioning through convenience capabilities, rapid decision-making support, and comprehensive quality control creates opportunities for emergency medicine applications through technology-enabled diagnostic advancement. Expected revenue pool: USD 16-21 million
Primary Classification: The market segments by assay type into Immunoturbidimetric Assay Kit, ELISA Kit, and Others categories, representing the evolution from manual colorimetric methods to advanced automated immunoassay platforms for comprehensive clinical diagnostic optimization.
Secondary Classification: Application segmentation divides the market into Hospitals and Laboratory sectors, reflecting distinct requirements for throughput capacity, analytical performance, and regulatory compliance standards.
Regional Classification: Geographic distribution covers Asia Pacific, Europe, North America, Latin America, and the Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by healthcare infrastructure expansion programs.
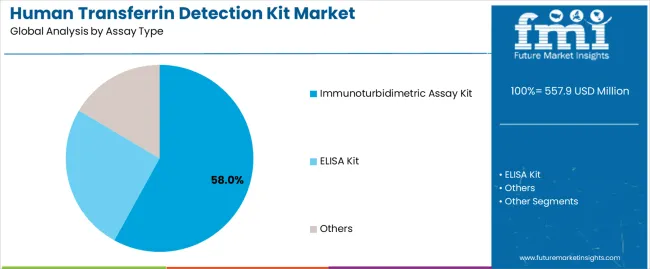
Market Position: Immunoturbidimetric assay kits command the leading position in the human transferrin detection kit market with approximately 58% market share through advanced automation features, including superior throughput capability, excellent precision performance, and analytical optimization that enable clinical laboratories to achieve optimal diagnostic results across diverse testing environments.
Value Drivers: The segment benefits from laboratory preference for automated testing systems that provide rapid turnaround times, reduced manual intervention, and long-term analytical stability without requiring significant workflow modifications. Advanced platform features enable multi-parameter integration, continuous operation capability, and compatibility with existing clinical chemistry analyzers, where analytical speed and operational efficiency represent critical laboratory requirements.
Competitive Advantages: Immunoturbidimetric systems differentiate through proven analytical reliability, consistent performance characteristics, and integration with modern laboratory automation processes that enhance operational effectiveness while maintaining optimal diagnostic accuracy suitable for diverse clinical applications.
Key market characteristics:
ELISA kit products maintain a significant 32.0% market share in the human transferrin detection kit market due to their exceptional flexibility and research application suitability. These platforms appeal to research laboratories requiring customizable protocols with comprehensive detection sensitivity for experimental applications. Market growth is driven by biomarker research advancement, emphasizing detection precision and protocol adaptability through microplate technology systems.
Alternative assay technologies hold approximately 10.0% market share, focusing on point-of-care testing formats and rapid diagnostic solutions. These platforms demand simplified operation processes for emergency settings requiring immediate diagnostic information with portable testing capabilities.
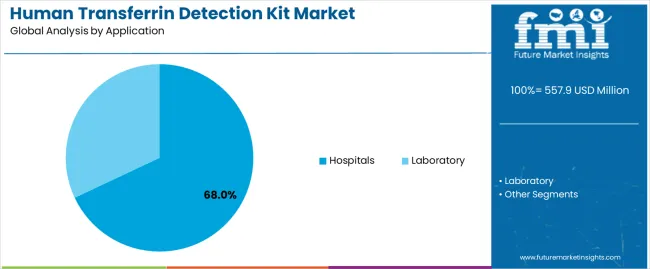
Market Context: Hospital applications dominate the human transferrin detection kit market with approximately 68% market share due to critical diagnostic requirements and increasing focus on iron deficiency assessment optimization, anemia diagnosis management, and clinical chemistry enhancement applications that minimize diagnostic delays while maintaining hospital laboratory standards.
Appeal Factors: Clinical laboratory directors prioritize analytical reliability, throughput capacity, and integration with laboratory automation infrastructure that enables coordinated testing across multiple diagnostic parameters. The segment benefits from substantial diagnostic technology investment and laboratory modernization programs that emphasize the acquisition of high-performance analytical systems for routine clinical applications.
Growth Drivers: Chronic disease management programs incorporate transferrin testing as standard requirements for nutritional assessment, while geriatric care advancement increases the need for comprehensive iron metabolism evaluation that complies with clinical guidelines and minimizes diagnostic uncertainty.
Market Challenges: Varying reimbursement policies and testing frequency guidelines may limit protocol standardization across different healthcare systems or clinical scenarios.
Application dynamics include:
Research laboratory applications capture approximately 32.0% market share through biomarker research requirements in pharmaceutical development, academic investigation operations, and protein quantification studies. These applications demand high-sensitivity detection platforms capable of operating in research environments while delivering exceptional analytical precision and experimental reproducibility characteristics.
| Parameter | Immunoturbidimetric Assay Kits | ELISA Kits | Other Assay Formats (Point-of-Care & Rapid Tests) |
|---|---|---|---|
| Market Share (2025) | ~58.0% | ~32.0% | ~10.0% |
| Core Application Segment | Hospital and clinical laboratories | Research and pharmaceutical studies | Emergency and decentralized testing |
| Analytical Strengths | High precision, automation compatibility, and reproducibility | High sensitivity and customization flexibility | Rapid turnaround and minimal sample requirement |
| Operational Requirements | Requires automated analyzers and standardized calibration systems | Manual or semi-automated setup; more operator input | Portable analyzers or lateral flow systems |
| Typical Turnaround Time | 10–15 minutes per assay cycle | 60–90 minutes per plate | <10 minutes per test |
| Cost Efficiency | High throughput reduces per-test cost | Higher reagent and labor cost per analysis | Variable, depending on platform type |
| Target User Base | Hospital laboratories, diagnostic networks | Academic research institutions, pharmaceutical R&D | Emergency care units, small clinical setups |
| Technology Trend (2030–2035) | Integration with laboratory information systems and AI-enabled quality control | Transition to multiplex ELISA platforms for biomarker discovery | Expansion into digital point-of-care diagnostics |
| Growth Opportunity Outlook | Stable, volume-driven growth through automation penetration | Niche but expanding through research-driven innovation | Emerging; driven by miniaturized testing and rapid diagnostics initiatives |
| Strategic Positioning Insight | Core market driver ensuring diagnostic standardization across hospitals | Research enabler that underpins biomarker validation studies | Frontier innovation area for decentralized healthcare delivery |
Growth Accelerators: Iron deficiency anemia prevalence drives primary adoption as human transferrin detection kits provide diagnostic assessment capabilities that enable clinicians to meet iron status evaluation standards without invasive testing, supporting patient care operations and treatment monitoring missions that require accurate biomarker quantification. Chronic disease burden accelerates market expansion as healthcare facilities seek comprehensive metabolic testing that maximizes diagnostic utility while maintaining analytical effectiveness during routine laboratory and specialty testing scenarios. Diagnostic technology investment increases worldwide, creating sustained demand for automated systems that complement traditional clinical chemistry methods and provide operational flexibility in complex laboratory environments.
Growth Inhibitors: Reagent cost considerations vary across healthcare systems regarding the pricing of immunoturbidimetric versus ELISA platforms, which may limit operational flexibility and market penetration in regions with budget constraints or cost-sensitive laboratory operations. Sample interference challenges persist regarding lipemia and hemolysis effects that may reduce analytical accuracy in compromised specimens, affecting diagnostic reliability and clinical interpretation. Market fragmentation across multiple analyzer platforms and reagent specifications creates compatibility concerns between different supplier systems and existing laboratory instrumentation.
Market Evolution Patterns: Adoption accelerates in hospital laboratories and clinical diagnostic sectors where throughput requirements justify automated system investments, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by healthcare infrastructure expansion and laboratory development. Technology development focuses on enhanced analytical sensitivity, improved interference resistance, and integration with laboratory automation platforms that optimize testing efficiency and diagnostic quality. The market could face disruption if alternative iron status biomarkers or point-of-care technologies significantly change the deployment of traditional transferrin testing in clinical applications.
The human transferrin detection kit market demonstrates varied regional dynamics with Growth Leaders including China (7.6% CAGR) and India (7.0% CAGR) driving expansion through healthcare infrastructure development and diagnostic laboratory network growth. Steady Performers encompass Germany (6.4% CAGR), Brazil (5.9% CAGR), and United States (5.3% CAGR), benefiting from established clinical laboratory industries and advanced diagnostic technology adoption. Mature Markets feature United Kingdom (4.8% CAGR) and Japan (4.2% CAGR), where specialized testing applications and quality-focused diagnostics support consistent growth patterns.
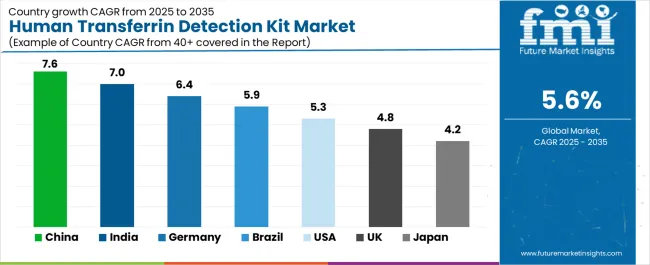
| Country | CAGR (2025-2035) |
|---|---|
| China | 7.6% |
| India | 7.0% |
| Germany | 6.4% |
| Brazil | 5.9% |
| United States | 5.3% |
| United Kingdom | 4.8% |
| Japan | 4.2% |
Regional synthesis reveals Asia-Pacific markets leading adoption through healthcare system modernization and diagnostic laboratory expansion, while European countries maintain steady growth supported by clinical guideline advancement and diagnostic standardization requirements. North American markets show moderate growth driven by chronic disease management applications and laboratory automation integration trends.
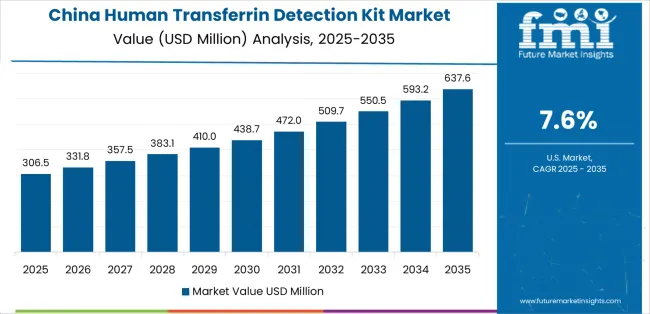
China establishes high-growth leadership through rapid healthcare infrastructure expansion and comprehensive diagnostic laboratory development, integrating human transferrin detection kits as standard diagnostic tools in hospital laboratory and clinical testing installations. The country's 7.6% CAGR reflects healthcare modernization initiatives promoting diagnostic capability and laboratory quality standards that mandate the use of validated immunoassay systems in hospital and medical center operations. Growth concentrates in major urban centers, including Beijing, Shanghai, and Guangzhou, where diagnostic adoption showcases integrated laboratory automation systems that appeal to hospital laboratories seeking advanced analytical capabilities and quality assurance solutions.
Chinese diagnostic manufacturers are developing cost-effective testing platforms that combine competitive pricing advantages with improved analytical features, including enhanced antibody specificity and automated analyzer compatibility. Distribution channels through hospital procurement networks and laboratory equipment suppliers expand market access, while quality certification programs for laboratory standardization support adoption across diverse hospital chemistry and clinical diagnostic segments.
Strategic Market Indicators:
In Mumbai, Delhi, and Bangalore, hospital laboratories and diagnostic centers are implementing human transferrin detection kits as standard analytical tools for iron metabolism assessment and anemia diagnosis, driven by increasing healthcare access and diagnostic infrastructure development programs that emphasize the importance of standardized testing capabilities. The market holds a 7.0% CAGR, supported by expanding hospital networks and diagnostic quality advancement initiatives that promote validated immunoassay systems for clinical laboratory and testing facilities. Indian laboratories are adopting immunoturbidimetric platforms that provide analytical reliability and throughput efficiency, particularly appealing in urban regions where diagnostic volume and quality standards represent critical operational requirements.
Market expansion benefits from growing clinical laboratory capabilities and international technology partnerships that enable domestic availability of diagnostic testing platforms for hospital laboratory and diagnostic center applications. Technology adoption follows patterns established in clinical chemistry analyzers, where automation capability and analytical quality drive procurement decisions and operational deployment.
Market Intelligence Brief:
Germany's diagnostic market demonstrates robust analytical capability with documented testing effectiveness in clinical chemistry operations and hospital laboratory integration through established quality management infrastructure. The country leverages laboratory expertise and regulatory compliance capability to maintain a 6.4% CAGR. Laboratory facilities, including Berlin, Munich, and Hamburg regions, showcase diagnostic installations where automated immunoassay platforms integrate with comprehensive laboratory information systems and quality control programs to optimize analytical effectiveness.
German laboratory operators prioritize analytical accuracy and quality assurance in platform selection, creating demand for advanced systems with comprehensive validation features, including precision verification and reference range studies. The market benefits from established clinical laboratory infrastructure and willingness to invest in quality-enhancing diagnostic technologies that provide analytical benefits and compliance with medical laboratory standards.
Market Intelligence Brief:
Brazil's market expansion benefits from growing healthcare access, including laboratory development in São Paulo and Rio de Janeiro, diagnostic infrastructure advancement, and government healthcare programs that increasingly require standardized diagnostic testing for clinical applications. The country maintains a 5.9% CAGR, driven by healthcare system development and increasing recognition of diagnostic quality benefits, including analytical standardization and testing reliability capabilities.
Market dynamics focus on cost-effective diagnostic solutions that balance analytical performance with affordability considerations important to Brazilian healthcare facilities. Growing healthcare awareness creates sustained demand for validated diagnostic platforms in laboratory infrastructure and healthcare modernization projects.
Strategic Market Considerations:
The USA market emphasizes advanced analytical features, including middleware integration and comprehensive laboratory information system connectivity that handles result management, quality tracking, and clinical decision support applications through unified healthcare platforms. The country holds a 5.3% CAGR, driven by chronic disease management advancement and laboratory efficiency optimization that support diagnostic technology integration. American clinical laboratories prioritize operational effectiveness with diagnostic platforms delivering consistent analytical performance through advanced immunochemistry and automation capabilities.
Technology deployment channels include major hospital systems, reference laboratories, and healthcare procurement programs that support professional applications for high-volume testing requirements. Laboratory information system integration capabilities with established healthcare platforms expand market appeal across diverse operational requirements seeking efficiency and quality benefits.
Performance Metrics:
In London, Manchester, and diagnostic centers, British hospital laboratories and clinical facilities are implementing advanced immunoassay platforms to enhance diagnostic quality capabilities and support modern laboratory operations that align with accreditation standards and clinical requirements. The UK market holds a 4.8% CAGR, driven by laboratory quality traditions and diagnostic efficiency upgrades that emphasize validated systems for clinical chemistry and hospital laboratory applications. British laboratories are prioritizing automated platforms that provide analytical consistency while maintaining quality compliance, particularly important in NHS facilities and accredited diagnostic laboratories.
Market expansion benefits from laboratory quality culture that mandates analytical validation capabilities in diagnostic specifications, creating sustained demand across the UK's hospital laboratory and diagnostic sectors, where analytical accuracy and regulatory compliance represent critical requirements. The healthcare framework supports automated testing adoption through quality standards and laboratory accreditation requirements that promote validated diagnostic platforms aligned with clinical laboratory specifications.
Strategic Market Indicators:
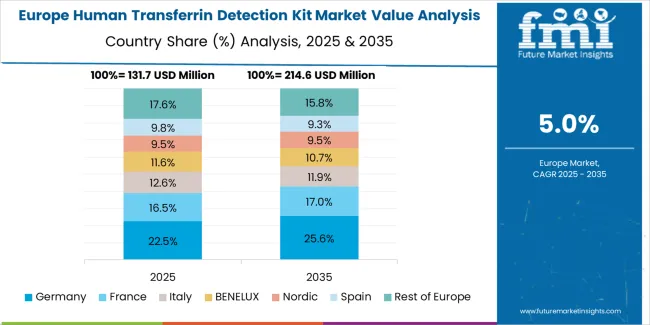
The European human transferrin detection kit market is projected to grow steadily over the forecast period. Germany is expected to maintain its leadership position with a 32.4% market share in 2025, supported by its advanced clinical laboratory infrastructure and major hospital networks across Berlin, Munich, and Hamburg regions.
France follows with a 26.8% share in 2025, driven by comprehensive laboratory quality programs and diagnostic technology development initiatives. Spain holds an 18.6% share in 2025 through hospital laboratory advancement and healthcare system modernization. The United Kingdom commands a 14.2% share, while Italy accounts for 8.0% in 2025. The Rest of Europe region is anticipated to maintain momentum attributed to increasing laboratory automation in Nordic countries and emerging Eastern European diagnostic facilities implementing quality testing programs.
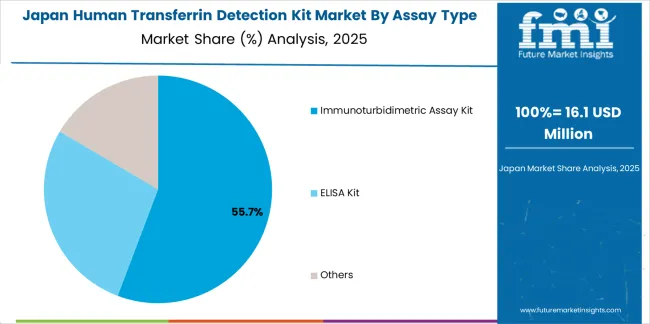
In Japan, the human transferrin detection kit market prioritizes immunoturbidimetric assay systems, which capture the dominant share of hospital laboratory and clinical testing installations due to their advanced features, including superior analytical precision and seamless integration with existing clinical chemistry infrastructure. Japanese laboratories emphasize quality, analytical reliability, and long-term performance excellence, creating demand for automated systems that provide consistent diagnostic capabilities and adaptive performance based on testing requirements and quality standards. Other assay formats maintain secondary positions primarily in specialized research applications and manual testing installations where ELISA platforms meet operational requirements without compromising analytical quality.
Market Characteristics:
In South Korea, the market structure favors international diagnostic manufacturers, including Thermo Fisher Scientific, Bio-Techne, and LifeSpan BioSciences, which maintain dominant positions through comprehensive product portfolios and established laboratory networks supporting hospital diagnostic and clinical testing installations. These providers offer integrated solutions combining validated immunoassay platforms with professional technical services and ongoing application support that appeal to Korean laboratories seeking reliable diagnostic systems. Local distributors and laboratory service providers capture a moderate market share by providing localized technical support and competitive pricing for standard clinical installations, while domestic manufacturers focus on specialized applications and cost-effective solutions tailored to Korean laboratory characteristics.
Channel Insights:
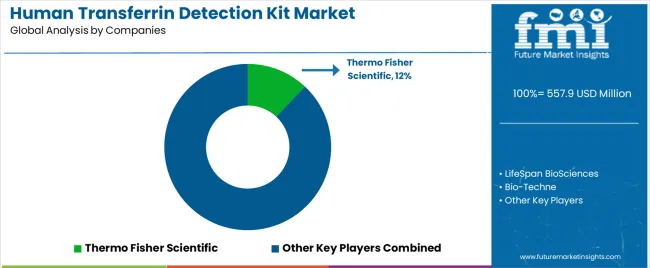
The human transferrin detection kit market operates with moderate concentration, featuring approximately 25-30 meaningful participants, where leading companies control roughly 45-50% of the global market share through established laboratory relationships and comprehensive diagnostic portfolios. Thermo Fisher Scientific maintains a leading position with approximately 12% market share through extensive immunoassay expertise and global distribution operations. Competition emphasizes analytical performance, regulatory compliance, and technical support rather than price-based rivalry.
Market Leaders encompass Thermo Fisher Scientific, Bio-Techne, and LifeSpan BioSciences, which maintain competitive advantages through extensive immunochemistry expertise, global laboratory distributor networks, and comprehensive quality validation capabilities that create customer loyalty and support premium pricing. These companies leverage decades of diagnostic development experience and ongoing research investments to develop advanced detection platforms with analytical sensitivity and operational reliability features.
Technology Innovators include Cygnus Technologies, Enzo, and Proteintech Group, which compete through specialized antibody technology focus and innovative detection platforms that appeal to laboratories seeking advanced analytical capabilities and research-grade quality. These companies differentiate through rapid product development cycles and specialized immunoassay application focus.
Regional Specialists feature companies like Creative Biolabs, EagleBio, and Xiamen Wiz Biotech, which focus on specific geographic markets and specialized applications, including custom assay development and research-grade reagents. Market dynamics favor participants that combine reliable analytical performance with comprehensive technical support, including validation assistance and quality documentation capabilities. Competitive pressure intensifies as traditional clinical chemistry manufacturers expand into specialized immunoassays, while emerging diagnostic companies challenge established players through innovative detection technologies and direct laboratory engagement platforms targeting modern clinical testing segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 557.9 million |
| Assay Type | Immunoturbidimetric Assay Kit, ELISA Kit, Others |
| Application | Hospitals, Laboratory |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 25+ additional countries |
| Key Companies Profiled | LifeSpan BioSciences, Thermo Fisher Scientific, Bio-Techne, Cygnus Technologies, Enzo, Proteintech Group |
| Additional Attributes | Dollar sales by assay type and application categories, regional adoption trends across Asia-Pacific, Europe, and North America, competitive landscape with diagnostic manufacturers and immunoassay suppliers, laboratory preferences for analytical accuracy and system reliability, integration with clinical chemistry analyzers and laboratory automation platforms, innovations in antibody technology and detection sensitivity, and development of quality programs with enhanced technical support and validation documentation capabilities. |
The global human transferrin detection kit market is estimated to be valued at USD 557.9 million in 2025.
The market size for the human transferrin detection kit market is projected to reach USD 962.0 million by 2035.
The human transferrin detection kit market is expected to grow at a 5.6% CAGR between 2025 and 2035.
The key product types in human transferrin detection kit market are immunoturbidimetric assay kit, elisa kit and others.
In terms of application, hospitals segment to command 68.0% share in the human transferrin detection kit market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Human Papilloma Virus Testing Market Size and Share Forecast Outlook 2025 to 2035
Human-Centric Lighting Market Size and Share Forecast Outlook 2025 to 2035
Human Identification Market Size and Share Forecast Outlook 2025 to 2035
Human Immunodeficiency Virus Type 1 (HIV 1) Market Size and Share Forecast Outlook 2025 to 2035
Humanoid Robot Market Size and Share Forecast Outlook 2025 to 2035
Humanized Mouse Model Market Size and Share Forecast Outlook 2025 to 2035
Human Milk Oligosaccharides Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Human Combinatorial Antibody Libraries (HuCAL) Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Human Growth Hormone (HGH) Treatment and Drugs Market Trends - Growth & Forecast 2025 to 2035
Human Augmentation Technology Market Growth - Trends & Forecast 2025 to 2035
Key Companies & Market Share in the Human Milk Oligosaccharides Sector
Human RSV Treatment Market Insights - Innovations & Forecast 2025 to 2035
Human Osteoblasts Market – Growth & Forecast 2024-2034
Human Capital Management Market
Human Anatomical Models Market
Human Machine Interface Market
UK Human Milk Oligosaccharides Market Trends – Size, Demand & Forecast 2025-2035
USA Human Milk Oligosaccharides Market Insights – Growth & Demand 2025-2035
ASEAN Human Milk Oligosaccharides Market Report – Size, Demand & Growth 2025–2035
Europe Human Milk Oligosaccharides Market Growth – Trends, Demand & Innovations 2025-2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA