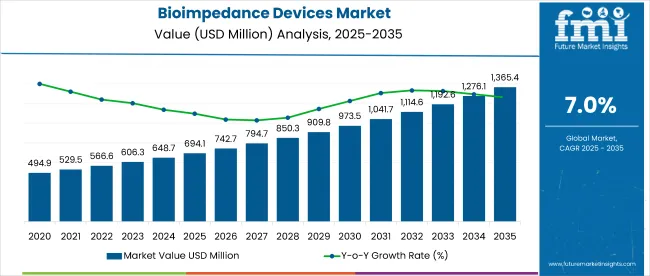
The global bioimpedance devices market is projected to grow from USD 694.1 million in 2025 to approximately USD 1,369.7 million by 2035. This reflects a compound annual growth rate (CAGR) of 6.7% over the forecast period. In 2024, demand for bioimpedance devices remained steady across nephrology, oncology, cardiology, and nutrition-based specialties. By 2025, adoption has accelerated due to their proven value in non-invasive diagnostics and patient monitoring.
Bioimpedance analysis (BIA) has become increasingly important in fluid status evaluation, dry weight determination, and body composition assessments. As healthcare systems prioritize preventive diagnostics and outpatient monitoring, these devices are gaining traction in both hospitals and home-care settings. In 2025, advanced BIA systems are being used to support dialysis planning, lymphedema tracking, and malnutrition screening. Their utility in chronic disease management and remote care frameworks is growing.
Multiple-frequency devices have been favored for their superior accuracy in distinguishing intracellular from extracellular fluid. In 2025, these devices are being integrated with software platforms that support longitudinal tracking and real-time alerts. Hospitals have shown a strong preference for wired models due to their reliable connectivity and compatibility with EHR systems. Wireless variants, though less dominant, are gaining share in sports medicine, wellness diagnostics, and post-surgical rehabilitation.
In 2024 and early 2025, several innovations were introduced to enhance usability. Device miniaturization, touchscreen interfaces, and cloud-enabled data logging are now standard in most high-end models. Companies such as ImpediMed, Bodystat, InBody, and Seca have focused on expanding product validation across clinical use cases. Some have partnered with research institutions to integrate AI-assisted interpretation, thereby supporting more informed diagnostics and personalized therapy planning.
The market’s momentum is being driven by structural trends in remote care, aging demographics, and chronic disease burden. As healthcare systems seek scalable, accurate, and patient-friendly tools, bioimpedance devices are positioned to play a critical role in future diagnostics and monitoring pathways.
| Attributes | Key Insights |
|---|---|
| Historical Size, 2024 | USD 650.5 million |
| Estimated Size, 2025 | USD 694.1 million |
| Projected Size, 2035 | USD 1,369.7 million |
| Value-based CAGR (2025 to 2035) | 6.7% |
Government regulations for bioimpedance devices focus on ensuring safety, quality, and clinical reliability before these products reach users. Most countries classify them as medium-risk medical devices, requiring formal registration, technical documentation, and post-market oversight. Regulatory authorities such as the FDA (U.S.), MDR (EU), CDSCO (India), and NMPA (China) mandate conformity to national standards, including labeling, electrical safety, and manufacturing quality systems.
New bioimpedance wearables are moving fluid assessment from spot-check tools to continuous, motion‑tolerant monitoring.
The above table presents the expected CAGR for the global Bioimpedance Devices Market over several semi-annual periods spanning from 2025 to 2035. In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 7.7%, followed by a slightly higher growth rate of 7.2% in the second half (H2) of the same decade.
| Particular | Value CAGR |
|---|---|
| H1 | 7.7% (2024 to 2034) |
| H2 | 7.2% (2024 to 2034) |
| H1 | 6.7% (2025 to 2035) |
| H2 | 6.2% (2025 to 2035) |
The above table presents the expected CAGR for the global Bioimpedance Devices Market over several semi-annual periods spanning from 2025 to 2035. In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 7.7%, followed by a slightly lower growth rate of 7.2% in the second half (H2) of the same decade.
Moving into the subsequent period, from H1 2025 to H2 2035, the CAGR is projected to decrease slightly to 6.7% in the first half and increase moderately at 6.2% in the second half. In the first half (H1) the market witnessed a decrease of -100.00 BPS while in the second half (H2), the market witnessed an decreases of -100.00 BPS.
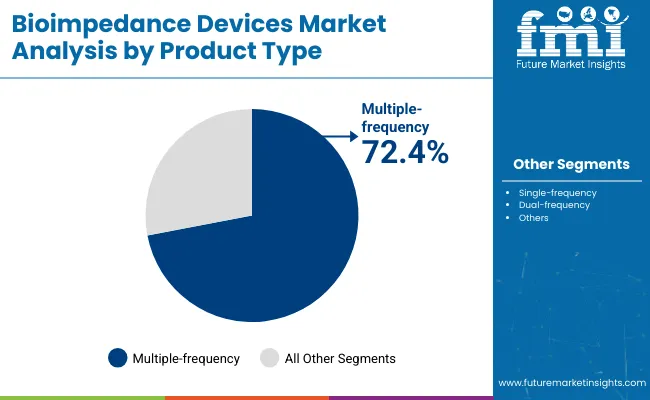
By product type, multiple-frequency bioimpedance devices are projected to hold 72.4% of the market share in 2025. These systems provide layered insights into intracellular and extracellular water content, which are vital for accurate fluid management, dialysis preparation, and clinical research. Their ability to scan at multiple frequencies allows more precise assessments, especially for patients with complex hydration or nutritional status.
Hospitals and academic institutes prefer this segment for its diagnostic reliability. Single-frequency devices, with a 27.6% share, continue to serve fitness and wellness users seeking cost-effective options. While still used in some clinical settings, their lower accuracy has reduced their popularity in critical care applications.
In 2024 and 2025, multiple-frequency devices saw upgraded software interfaces, enhanced calibration features, and easier data transfer protocols. Integration with telehealth systems has made them more relevant for outpatient and chronic care models. As data-driven diagnostics take center stage, the demand for robust and multifrequency-enabled bioimpedance analysis is expected to grow substantially in both clinical and sports performance environments.
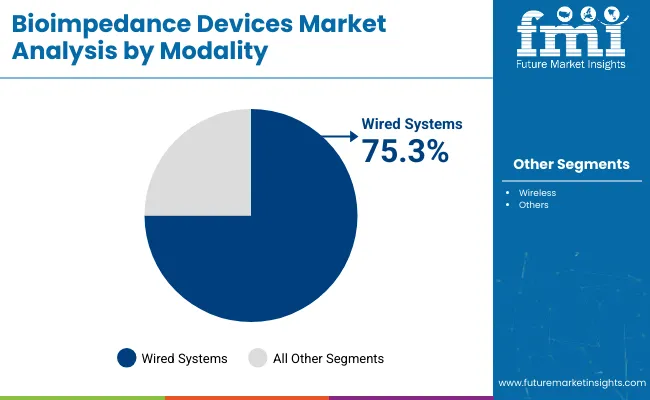
By modality, wired bioimpedance devices are forecast to command 75.3% of the market in 2025. Their reliability, seamless integration into hospital systems, and consistent power delivery make them the preferred choice for intensive clinical settings. Wired units support higher data throughput and real-time transfer, allowing them to be embedded within electronic health record (EHR) platforms and diagnostic dashboards.
In 2024 and 2025, hospitals favored wired devices for fluid management in dialysis, intensive care, and cardiac rehab programs. Wireless bioimpedance devices, with a 24.7% share, are steadily growing in fitness tracking, mobile diagnostics, and home care use. These devices offer better flexibility and patient comfort but face limitations in data fidelity and signal stability under certain clinical conditions.
Wireless systems have found success in outpatient settings, especially for monitoring weight changes, lymphatic conditions, or post-operative recovery. While they are advancing in algorithmic precision, wired systems continue to lead the segment based on institutional preference and integration capabilities. Future growth in wireless systems will likely hinge on continued improvements in signal reliability, cloud-syncing efficiency, and regulatory clearances.
The Growing Obese Population Worldwide is a Significant Driver for the Bioimpedance Devices Market
Growth of the obesogenic population is one such factor that drives the bioimpedance devices market globally. Key concerns falling under public health would be obesity, a condition in which the body excessively deposits fat in relation to other tissues, which, in turn, gives rise to risks for cardiovascular diseases, diabetes, and musculoskeletal disorders.
Bioimpedance devices offer clinicians useful applications in the evaluation of body composition and the monitoring of metabolic health, in addition to enabling them to provide personalized interventions in the course of its management.
Noninvasive methods of assessment of body composition, such as bioimpedance devices, include the assessment of fat mass, lean body mass, and total body water content. Such information is important in understanding the distribution of body fat and muscle mass, which differ so much among people with obesity.
Metabolic abnormalities with obesity, including insulin resistance, dyslipidemia, and hypertension, are managed together in most cases under the umbrella of the metabolic syndrome. Metabolic risk factors and cardiovascular disease risk are closely associated with visceral obesity and can be measured using impedance devices. These metabolic markers can be used for regular monitoring, and intervention by healthcare professionals to try and improve metabolic health and decrease related complications associated with the risk of obesity.
Bioimpedance technology supports all the weight management programs through the discreet measurement of changes in body composition over time. It allows the health provider to evaluate interventions on dietetics, programs for exercising, and the use of drugs regarding their effects on the reduction of fat mass and the preservation of body muscle tissue. Real-time data from bioimpedance devices allows a person with obesity to track progress in order to make health and wellness decisions.
The Increasing Prevalence of Chronic Kidney Disease is a Compelling Driver for the Bioimpedance Devices Market
The rising prevalence of chronic kidney diseases worldwide acts as the key growth driver for the market of bioimpedance devices. Chronic kidney disease is defined as the progressive loss of appropriate functioning of one's kidneys over a long period.
It results in complications in the balance of water and electrolytes, leading to cardiovascular complications. It is thus of paramount importance that on its part, bioimpedance monitoring be able to offer optimum clinical care to patients with chronic kidney disease by allowing early stage detection, continuous monitoring, and individualized management.
On top of it, according to the American Kidney Fund, kidney disease affects as many as 37 million Americans, with nearly 808,000 living with kidney failure. More than 557,000 Americans are on dialysis. The prevalence of kidney disease is rising rapidly and currently affects more than 1 in 7-14 percent-of American adults.
Fluid status remains one of the main clinical problems in the care of patients with CKD. Fluid overload or depletion has complications, including hypertension and heart failure. In these regards, bioimpedance devices offer techniques of fluid distribution in a noninvasive way within the body, such as by measurements of the extracellular and intracellular fluid compartments. With the aid of bioimpedance technology, healthcare providers are well-placed to make informed decisions for the management of CKD by deriving overall data points that include fluid status, body composition, and nutritional metrics.
The High Cost of Consumables & Accessories Associated with Bioimpedance Devices Presents a Multifaceted Challenge for the Market
High consumable and accessory cost associated with bioimpedance devices is a factor for the colossal negative impact on the market from the perspective of the health care providers, health care facilities, and finally the patient. Despite bioimpedance being an important biomedical technology for applications ranging from body composition analysis, fluid monitoring, and metabolic assessment, the conventional cost of consumables and accessories can affect two fronts: both the financial burden and logistical handling in a healthcare ecosystem.
The cost burden of consumables and accessories for bioimpedance devices would indirectly affect patient care related to advanced diagnostic and monitoring technology. Such providers will have to make really hard decisions on how often their equipment will be used, when it will be serviced, and when the consumables should or could be replaced.
These may result in delays in patient evaluation or suboptimal care for chronic conditions, such as renal failure or heart failure. In some cases, high costs contribute to limiting access to bioimpedance technology in general, especially in under-resourced localities or low-income regions, where resources in health are already scant.
The bioimpedance devices require consumables and accessories as well as technical support for maintaining, calibrating, and the efficient performance of the device in order to increase its life span. The costs of the consumables and accessories are relatively high. Other costs also include the training and education of staff, servicing the equipment, and the maintenance and keeping up with the use of the regulatory standards.
As Research and Innovation Continue to Advance, Bioimpedance Devices Are Poised to Play a Pivotal role in Transforming Cancer Management Practices
Early cancer diagnosis is one of the most promising applications of bioimpedance technology in oncology. Measured with bioimpedance devices, the electrical properties include resistance and reactance, which are vastly different between the healthy and malignant tissues. Thus, bioimpedance devices hold the task of early detection of malignancies, often before visible size with traditional imaging techniques can be achieved.
This ability is most useful for cancers that are hard to identify at an early stage, such as breast cancer, prostate cancer, and colorectal cancer. Here, the bioimpedance devices take a non-invasive and non-intimidating approach compared to the more invasive ways of diagnosis, such as biopsies or extensive imaging studies. Use-easy and fast results from bioimpedance devices allow earlier diagnosis, critical to improving survival rates and reducing the overall burden of cancers.
Bioelectrical impedance devices offer a very critical role is during the monitoring of cancer patients outlined during their treatment. Chemotherapy and radiation therapy, among other cancer treatments, impact massive changes in the body composition, massively characterized by fluid balance, muscle mass, and the overall health status of tissue. The bioimpedance technology enables continuous, non-invasive measurement of these parameters and gives real-time information that can be used in evolving treatment planning as necessary.
The measures can be helpful in assessing patient recovery in general and, in particular, in body composition changes that signify the reemergence of the disease or the development of some health problems related to past treatments.
Biological impedance technology is thus playing a significant and critical role, varying from early diagnosis and treatment monitoring to post-treatment surveillance throughout the stages of cancer care.
The global bioimpedance devices industry recorded a CAGR of 4.5% during the historical period between 2020 to 2024. The growth of bioimpedance devices industry was positive as it reached a value of USD 650.5 million in 2024 from USD 546.3 million in 2020.
Bioimpedance devices gives detailed information on the mass of fat, muscles, and the level of water, very critical information in developing personalized wellness programs. A wellness center, powered by this bioimpedance, enables advisors to give specific suggestions on nutrition, exercise, and changes to be made in one's lifestyle with facts backing such advice. This will not only help in enhancing the efficacy of wellness programs but will also go a long way in enriching customer satisfaction by showing measurable progress in health journey of an individual.
Bioimpedance technology is ideal for weight loss clinics since it provides the accurate measurement of key metrics, which include body fat percentage and muscle mass and hydration status. Because measurements using bioimpedance are non-invasive, it is easy to track frequently-without discomfort-whether the client is on the right track of their weight management plan.
Bioimpedance devices at rehabilitation centers are very instrumental in monitoring a patient's recovery, especially muscle mass and hydration levels during the course of physical therapy. This data ensures that rehabilitation programs are effective and the patient progresses safely and efficiently. With wellness, weight management, and rehabilitation programs requiring accurate individual data, the demand for bioimpedance devices is likely to grow in these sectors, further fueling the market.
Tier 1 companies comprise market leaders with a market revenue of above USD 100 million capturing significant market share of 66.5% in global market. These companies invest significantly in advanced research and development to innovate new technologies that enhance treatment efficacy and improve patient outcomes.
Expanding global market presence is another key strategy, often achieved through strategic partnerships, acquisitions, and establishing strong distribution networks. Prominent companies within tier 1 include General Electric Company, Omron Corporation and Fresenius Medical Care.
Tier 2 companies include mid-size players with revenue of USD 50 to 100 million having presence in specific regions and highly influencing the local market and holds around 15.2% market share. These companies often differentiate themselves through niche product offerings or specialized services that cater to specific segments of the market.
Leveraging partnerships with healthcare providers and distributors helps expand their market reach and improve accessibility to their products. Emphasizing cost-efficiency in manufacturing and operations enables tier 2 companies to offer competitive pricing without compromising on quality. Prominent companies in tier 2 include ImpediMed Limited, SELVAS AI Inc., Tanita Corporation and RJL Systems, Inc.
Finally, Tier 3 companies, such as Maltron International Ltd., Bodystat Limited and SMT Medical GmbH are essential for the market. They specialize in specific products and cater to niche markets, adding diversity to the industry. Overall, while Tier 1 companies are the primary drivers of the market, Tier 2 and 3 companies also make significant contributions, ensuring the Bioimpedance Devices Market remains dynamic and competitive.
The section below covers the industry analysis for the bioimpedance devices market for different countries. Market demand analysis on key countries in several regions of the globe, including North America, Asia Pacific, Europe, and others is provided. The United States is anticipated to remain at the forefront in North America, with higher market share through 2035. In Asia Pacific, China is projected to witness a CAGR of 7.2% by 2035.
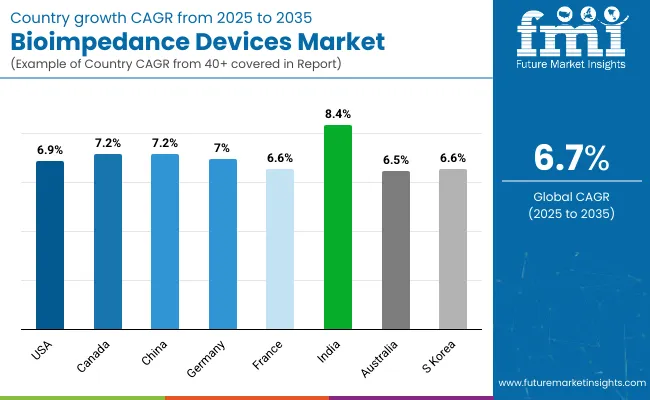
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| USA | 6.9% |
| Canada | 7.2% |
| China | 7.2% |
| Germany | 7.0% |
| France | 6.6% |
| India | 8.4% |
| Australia | 6.5% |
| South Korea | 6.6% |
USA Bioimpedance Devices Market is poised to exhibit a CAGR of 1.5% between 2025 to 2035. Currently, it holds the highest share in the North American market, and the trend is expected to continue during the forecast period.
The USA health care system is described as one that highly adapts to and invests in technology. It allows an environment in which the introduction and testing of advanced medical devices are possible, such as bioimpedance technology.
Whether hospitals, clinics, or research centers, the health facilities of the United States are efficient in adopting innovations for solutions related to health, creating a momentum in the demand for bioimpedance devices in medical specializations across the board.
This can be amply illustrated by the fact that obesity, diabetes, and cardiovascular conditions are highly prevalent in the USA, thus, accurate but non-invasive diagnostic tools, such as bioimpedance devices, are needed and relevant. They include body composition, fluid status, and metabolic health assessment in order to clinically indicate management strategies of a subject.
India holds around prominent share of the South Asia & Pacific bioimpedance devices industry. India’s market is anticipated to grow at a CAGR of 8.4% throughout the forecast period.
India's strengthening manufacturing and innovation has also resulted in a robust export market for bioimpedance devices. Increasingly, Indian manufacturers are shipping their products to various regions, including Africa, the Middle East, Southeast Asia, and even developed markets such as Europe and North America.
The competitive pricing has come on top of an increasingly sound quality reputation that has served to make Indian bioimpedance devices quite attractive in these markets.
Moreover, Indian companies form strategic joint ventures with international firms in an attempt to utilize the foreign experience and global networks in enhancing their market presence. The tie-ups would make it easier for Indian firms to enter new markets and further expand their global presence in the sector of bioimpedance devices.
Germany’s Bioimpedance Devices Market is poised to exhibit a CAGR of 7.0% between 2025 to 2035. Currently, it holds the highest share in the Asia Pacific market, and the trend is expected to continue during the forecast period.
The demographic change Germany is facing and the increase in chronic diseases, like diabetes, cardiovascular diseases, and obesity, underline the need for sophisticated diagnostic and monitoring tools-for example, bioimpedance devices.
Because devices of this kind are to assist in supporting body composition, fluid status, and metabolic health assessment, two critical tasks associated with early detection and application of personalized treatment strategies that can bring about improvement in patient outcomes.
That helps ensure product safety, efficacy, and conformance with EU requirements at the same time. As the German regulatory environment is actually building up confidence amongst health care providers in these technologies' proper blend, most manufacturers try availing this opportunity for proper market entry with their bioimpedance devices into Germany and all other EU markets.
Substantial investments are seen in the bioimpedance devices industry towards research and development in order to drive innovations by differentiating products through advanced features and improved patient outcomes. Another key strategic focus of these companies is to actively look for strategic partners to bolster their product portfolios and expand their global market presence.
Recent Industry Developments in Bioimpedance Devices Market:
| Report Attributes | Details |
|---|---|
| Current Total Market Size (2025) | USD 694.1 million |
| Projected Market Size (2035) | USD 1,369.7 million |
| CAGR (2025 to 2035) | 6.7% |
| Base Year for Estimation | 2024 |
| Historical Period | 2020 to 2024 |
| Projections Period | 2025 to 2035 |
| Quantitative Units | USD million for value and million units for volume |
| Product Types Analyzed (Segment 1) | Multi-frequency, Single-frequency, Dual-frequency |
| Applications Analyzed (Segment 2) | Whole-body Measurement, Segmental Body Measurement |
| Regions Covered | North America; Latin America; Western Europe; Eastern Europe; South Asia and Pacific; East Asia; Middle East & Africa |
| Countries Covered | United States, Canada, Germany, United Kingdom, France, Japan, China, India, South Korea, Brazil |
| Key Players Influencing the Market | Bioparhom, Akern, Biodynamics Corporation, Tanita Corporation, Biotekna, Omron Corporation, InBody, EVOLT 360, RJL Systems, Fook Tin Group Holding Ltd |
| Additional Attributes | Growing demand for body composition diagnostics, Rising adoption of multi-frequency systems, Expansion in wellness and clinical use cases |
| Customization and Pricing | Customization and Pricing Available on Request |
In terms of product, the industry is divided into multi-frequency, single-frequency and dual-frequency.
In terms of modality, the industry is segregated into wired bioimpedance and wireless bioimpedance.
In terms of application, the industry is segregated into whole-body measurement and segmental body measurement.
Key countries of North America, Latin America, Western Europe, Eastern, South Asia and Pacific, East Asia and Middle East and Africa (MEA) have been covered in the report.
The global bioimpedance devices market is projected to witness CAGR of 6.7% between 2025 and 2035.
The global bioimpedance devices industry stood at USD 694.1 million in 2025.
The global bioimpedance devices industry is anticipated to reach USD 1,369.7 million by 2035 end.
India is set to record the highest CAGR of 6.7% in the assessment period.
The key players operating in the global bioimpedance devices industry include Bioparhom, Akern, Biodynamics Corporation, Tanita Corporation and Omron Corporation are the key manufacturers of Bioimpedance Devices.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Bioimpedance Spectroscopy Market – Trends & Forecast 2025 to 2035
Global Bioimpedance Analyzer Market Insights – Trends & Forecast 2024-2034
FBAR Devices Market
Snare devices Market
C-Arms Devices Market Size and Share Forecast Outlook 2025 to 2035
Timing Devices Market Analysis - Size, Growth, & Forecast Outlook 2025 to 2035
Spinal Devices Market Size and Share Forecast Outlook 2025 to 2035
Hearing Devices 3D Printing Market Size and Share Forecast Outlook 2025 to 2035
Medical Devices Market Size and Share Forecast Outlook 2025 to 2035
Network Devices Market Size and Share Forecast Outlook 2025 to 2035
Medical Devices Secondary Packaging Market Analysis by Material and Application Through 2035
Hearable Devices Market Size and Share Forecast Outlook 2025 to 2035
Lab Chip Devices Market Size and Share Forecast Outlook 2025 to 2035
Orthotic Devices, Casts and Splints Market Size and Share Forecast Outlook 2025 to 2035
Lacrimal Devices Market Size, Trends, and Forecast 2025 to 2035
Global Ablation Devices Market Trends - Growth, Innovations & Forecast 2025 to 2035
Orthotic Devices, Splints & Orthopedic Braces Market Analysis - Trends & Forecast 2024 to 2034
Ear Tube Devices Market
Pathology Devices Market Size and Share Forecast Outlook 2025 to 2035
Neurotech Devices Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA