The CGRP Inhibitors industry is valued at USD 3.92 billion in 2025. As per FMI's analysis, the CGRP inhibitors industry will grow at a CAGR of 12.4% and reach USD 12.27 billion by 2035. The Calcitonin Gene-Related Peptide (CGRP) inhibitors industry is one of the largest and fastest-growing pharmaceutical sectors and is anticipated to witness continued expansion through the next decade.
This growth is due to the increasing prevalence of migraine and cluster headaches, advances in biologic therapies, and heightened awareness of the availability of specialized migraine treatments.
In 2024, the industry for CGRP (Calcitonin Gene-Related Peptide) witnessed significant developments shaping its trajectory. The launch of biosimilars and generics had a profound impact on the sector dynamics by providing affordable substitutes for branded CGRP inhibitors. This development improved patient access to migraine and cluster headache therapies, especially in areas with scarce healthcare resources.
New treatments have become available, clinical practice has evolved, and excitement and new hopes have come for the prevention and treatment of migraines through novel mechanisms of action with greater efficacy and tolerability compared to older options.
As awareness among patients and physicians grows over time, CGRP inhibitors are destined to be one of the cornerstone therapies in the management of migraine, altering the landscape of therapy and increasing the quality of life for millions of patients worldwide.
Key Market Insights
| Metric | Key Insights |
|---|---|
| Industry Size (2025E) | USD 3.92 billion |
| Industry Size (2035F) | USD 12.27 billion |
| CAGR (2025 to 2035) | 12.4% |
CGRP inhibitors' sector is increasing at a speedy rate, pushed by rising diagnosis of migraines and robust clinical performance of such targeted therapies. Pharma firms developing innovative or biosimilar products gain, while established migraine drug producers lose industry position. Expanded access and results in the real world continuing to impress, CGRP inhibitors become the new gold standard in migraines.
Accelerate Differentiated R&D
Invest in next-generation CGRP products or delivery systems (e.g., long-acting injectables or oral fixed combinations) to keep ahead of biosimilar pressure and enhance patient compliance.
Grow Market Access & Reimbursement Footprint
Closely align with emerging payer expectations and real-world evidence requirements to secure wider reimbursement, especially in new sectors and underpenetrated territories.
Strengthen Strategic Alliances & Distribution
Seek strategic alliances with specialty pharmacies, neurology networks, and digital health platforms to increase patient access, simplify distribution, and foster brand loyalty prior to wider competition.
| Risk | Probability - Impact |
|---|---|
| Pricing Pressure from Biosimilars | High - High |
| Reimbursement Delays or Restrictions | Medium - High |
| Safety Concerns or Regulatory Setbacks | Low - High |
| Priority | Immediate Action |
|---|---|
| Expand Global Access | Run feasibility on tiered pricing models and local manufacturing partnerships |
| Strengthen Clinical Positioning | Initiate KOL and provider feedback loop on unmet needs and real-world outcomes |
| Accelerate Industry Penetration | Launch a pilot program for specialty pharmacies and digital channel partners |
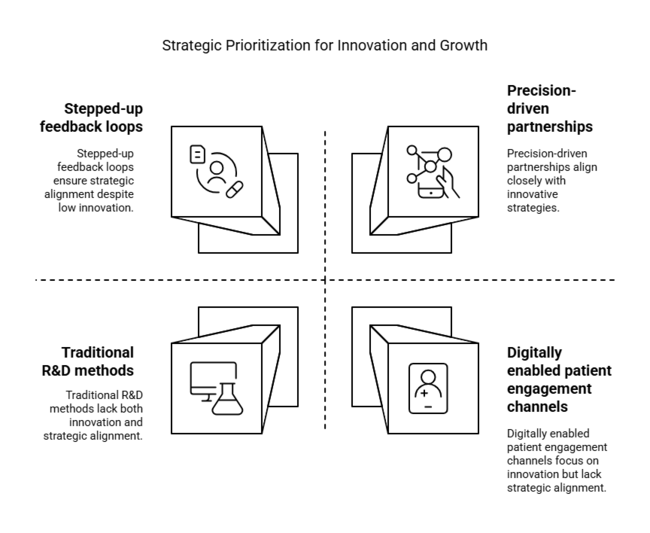
To stay ahead, the company must immediately shift to differentiated product innovation, global access strategies, and precision-driven partnerships. This report highlights a transition away from pure R&D-driven growth to value execution where payer alignment, real-world data, and targeted distribution will identify industry winners.
The roadmap now needs to focus on stepped-up feedback loops with prescribers, investment in adaptive price models for new sectors, and the deployment of and deploy digitally enabled patient engagement channels.
Regional Variance:
High Variance:
Convergent and Divergent ROI Views:
Consensus:
Variance:
Shared Challenge:
Regional Differences:
Manufacturers:
Prescribers:
Patients:
Alignment:
Divergence:
Key Variances:
Strategic Insight:
No single model will scale globally. Tailored strategies-injectables in the US, reimbursement agility in Europe, and cost-optimized oral options in Asia-are essential for long-term competitive edge.
| Countries/Region | Policy & Regulatory Impact |
|---|---|
| United States |
|
| Western Europe (EU) |
|
| Germany |
|
| France |
|
| United Kingdom |
|
| Japan |
|
| South Korea |
|
The USA sector for CGRP sales is projected to grow at a CAGR of around 7.8% during the forecast period 2025 to 2035. This increase is due to the high prevalence of migraine diseases, which affect approximately 12% of the population.
CGRP inhibitors like erenumab and fremanezumab became available, the way we treat migraines has changed because these drugs target specific processes in the body and are generally safe, giving patients new hope. The American Headache Society has actually endorsed these therapies, leading to further acceptance among healthcare providers.
The United Kingdom’s CGRP inhibitors sales are expected to grow at a CAGR of 8.1% during the coming 10 years. In the UK, CGRP inhibitor uptake has been slower than that in the USA, primarily because of rigorous cost-effectiveness evaluations by the National Institute for Health and Care Excellence.
These evaluations have impeded widespread use by healthcare professionals, migraine patients, and payers in a segment populated by numerous promising new treatments, the understanding of the potential unmet need in this space is growing, and as the effects of migraine management become more significant, and so will the demand for treatments as it matures over time.
The CGRP inhibitors sector in sales is likely to grow at a noteworthy CAGR of 8.0% during the forecast period of 2025 to 2035. The deliberate integration of CGRP inhibitors into clinical practice is the result of the French healthcare system's emphasis on assessment of therapeutic value and price negotiation.
Clinical proof of the efficacy of these treatments grows and patient demand rises as the industry is set for steady growth. Stakeholders will be critical in generating conditions for sector access, requiring collaborations between pharmaceutical companies and healthcare authorities to work through the difficulties of pricing and reimbursement.
The CGRP inhibitors sales in Germany is expected to witness a CAGR of around 8.5% over the forecast period. Germany's organized method of adding new medical treatments, along with a strong emphasis on how well they work and their costs, has influenced how quickly CGRP inhibitors are being used in medical practice.
However, Germany's extensive healthcare system and the presence of influential pharmaceutical companies make it well-positioned for sector expansion. Additional real-world data emerges confirming the effectiveness of CGRP inhibitors, their uptake is only likely to rise, underpinned by favorable reimbursement policies.
In Italy, between 2025 and 2035, the CGRP inhibitors sales are expected to expand with a CAGR of approximately 8.3%. This fragmentation has left the drug sector impacted by regional variability in drug approval and reimbursement across the Italian healthcare system.
Greater awareness of migraine management and innovative therapies will push demand upward. Streamlining of the approval process and ensuring that access to the products is uniform across geographies will be critical to achieving the potential growth of the sector.
The South Korean CGRP inhibitors sales is poised to grow at a significant CAGR of 12.8% over the forecast period of 2025 to 2035. The country's advanced medical infrastructure and rapid adoption of new technologies for health care reinforce this trend.
Continuing improvements in drug development and delivery systems, which optimize the effectiveness, convenience, and patient compliance associated with CGRP inhibitors, remain critical in supporting the growth of the sector. Increasing prevalence of migraine disorders and availability of novel therapeutics that use for managing migraine are anticipated to propel demand over the forecast period.
The CGRP inhibitors sales industry of Japan is anticipated to generate a CAGR of 13.2% during a forecast period. The favorable reimbursement policies and coverage under the national health insurance system are two of the factors contributing to patient access to CGRP inhibitors in the sector.
Increased awareness of migraine among the Japanese population also increases the use of such treatments. The presence of major pharmaceutical companies actively involved in research and development accelerates the industry growth.
The CGRP inhibitors sales are expected to grow in China at a strong CAGR of 13.5% by 2035. This enormous increase could be attributed to the country's growing healthcare infrastructure and rising disposable income, which have enabled people to afford better healthcare facilities.
There is a significant segment opportunity with a large patient pool suffering from migraine disorders. The Chinese government's efforts to promote innovative medical treatment and the increasing number of global pharmaceutical companies are also expected to facilitate industry growth.
The sales for CGRP inhibitors in Australia and New Zealand is expected to grow at a CAGR of over 8.5% between 2025 and 2035. High standards of health care and strong professional traditions of embracing new medical treatments. Increased awareness among healthcare professionals and patients, along with the rising prevalence of migraine disorders, drive industry growth.
Moreover, favorable reimbursement and the availability of specialized headache clinics further add to the growing usage of CGRP inhibitors in these sectors.
The sales for CGRP inhibitors in India are exceeding the global CAGR of 12.4% during the same period. The robust growth prospects are mainly attributed to growing recognition of migraine as a chronic neurological disorder, increasing stress levels in urban dwelling, and improvement in healthcare penetration.
CGRP inhibitors are still in early stages of penetration in the Indian sector. The need for effective prophylactic treatments is considerable, with many patients unable to prevent their headaches with conventional migraine therapies (including triptans, NSAIDs and beta-blockers).
The analysis estimates small molecule CGRP inhibitors to be the most profitable class in the CGRP therapeutics space in terms of revenue during 2025 to 2035 with a robust CAGR of 13.8%, overtaking large molecules during this period. The most significant contributor to this expansion is the growing use of oral gepants, which provide greater convenience, ease of administration, and wider access via pharmacies and telehealth platforms.
These drugs are appealing to a wider patient population since they address both the acute and preventive treatment requirements. They are also commercially attractive due to their lower production costs and rapid onset of action. They can be expensive, logistically complicated to administer, and pose tighter reimbursement barriers, limiting their broad adoption, particularly in emerging segments.
The preventive migraine treatment segment is expected to generate the highest revenue in the CGRP inhibitors landscape between 2025 and 2035, with an approximate CAGR of 14.2%. The robust growth is primarily attributable to the increasing prevalence of chronic migraine cases worldwide and the growing patient/physician inclination towards long-term management rather than episodic treatment.
Preventive treatments, especially CGRP-targeting monoclonal antibodies and new oral gepants like atogepant, have shown to be very effective in reducing the number of migraine days each month, which significantly improves patients’quality of life and daily functioning.
The oral route of administration is projected to be the most profitable segment of the CGRP inhibitors industry during the period between 2025 to 2035, with a CAGR of nearly 14.6%, followed by nasal and injectable delivery formats. This increase is mainly due to more patients wanting easy-to-use treatments that they can take themselves, especially the small molecule CGRP antagonists rimegepant and atogepant.
These treatments are rapidly utilized for acute and preventive migraine therapy with strong efficacy profiles and growing access through telehealth and virtual pharmacy. Moreover, it decreases reliance on healthcare professionals, thus leading to cost-efficiency and scalability, particularly in the emerging sectors.
The retail pharmacies end-user segment is anticipated to register the highest CAGR of 13.9% during the forecast period (2025 to 2035), and the CGRP inhibitors general industry is expected to be the highest-earning segment during 2025 to 2035. This is primarily due to the emergence of oral CGRP treatments that do not require specialist oversight or complex storage arrangements.
Retail pharmacies provide an unmatched access point for patients seeking both preventive and acute migraine treatment, making them the most accessible option in urban and semi-urban settings. Moreover, over-the-counter pharmaceutical models and the extension of pharmacist-prescribing rights across several sectors (e.g., North America, Europe) mean retail pharmacies are emerging as the lead channel for CGRP therapeutics.
The leading pharmaceutical contenders are competing on several strategic fronts in the CGRP inhibitors marketplace-pricing innovation, product differentiation, global expansion, and strategic alliances. AbbVie, Eli Lilly, Amgen, and Teva are using aggressive pricing models in sectors where they want to establish an early lead including places like Asia and Latin America that have cost-sensitive health-care systems.
On the upside, there are companies laying bets on novel CGRP-targeting molecules (oral small molecule antagonists (gepants), long-acting monoclonal antibodies, etc.) in great numbers. R&D efforts are multi-pronged, addressing both indications on efficacy-cluster headaches, medication overuse headaches-and even non-headache indications for neurological conditions across the CHPR pathways.
The industry is segmented into Small Molecule, Large Molecule
The industry is segmented into Preventive Migraine Treatment, Acute Migraine Treatment
The industry is segmented into Oral, Nasal, Injectables
The industry is segmented into Hospitals, Specialty Clinics, Mail Order Pharmacies, Retail Pharmacies
The industry is segmented into North America, Latin America, Europe, East Asia, South Asia, Oceania, and the Middle East & Africa
A class of drugs that block the calcitonin gene-related peptide pathway, which is involved in migraine attacks by causing blood vessels to dilate and carrying pain signals in Calcitonin gene-related peptide (CGRP) inhibitors.
Unlike older treatments, which often work to target symptoms once they have started, CGRP inhibitors can be deployed preventively and provide more specificity with fewer systemic side effects.
Yes, there are oral formulations (eg, the gepants) and injectables (eg, the monoclonal antibodies) of CGRP therapies available that provide patients and providers with options for flexible treatment.
Strong reimbursement infrastructure in North America and parts of Western Europe is enabling them to be the main lights at the present income points, while South Asia and Latin America are emerging zones for potential growth.
Significant players include AbbVie (ABBV), Amgen (AMGN), Eli Lilly (LLY), Teva (TEVA) and Pfizer (PFE) who have launched or are developing CGRP-focused therapies featuring novel delivery systems.
Table 01: Global Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 02: Global Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 03: Global Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 04: Global Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 05: Global Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Region
Table 06: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 07: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 08: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 09: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 10: North America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 11: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 12: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 13: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 14: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 15: Latin America Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 16: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 17: Europe Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 18: Europe Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 19: Europe Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 20: Europe Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 21: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 22: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 23: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 24: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 25: East Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 26: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 27: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 28: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 29: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 30: South Asia Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 31: Oceania Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 32: Oceania Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 33: Oceania Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 34: Oceania Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 35: Oceania Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Table 36: Middle East & Africa Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Country
Table 37: Middle East & Africa Market Value (US$ Million) Analysis 2018 to 2022 and Forecast 2023 to 2033, by Molecule
Table 38: Middle East & Africa Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Treatment Type
Table 39: Middle East & Africa Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Route of Administration
Table 40: Middle East & Africa Market Analysis 2018 to 2022 and Forecast 2023 to 2033, by Distribution Channel
Figure 01: Global Market Value (US$ Million) Analysis, 2018 to 2022
Figure 02: Global Market Forecast & Y-o-Y Growth, 2023 to 2033
Figure 03: Global Market Absolute $ Opportunity (US$ Million) Analysis, 2022 to 2033
Figure 04: Global Market Value Share (%) Analysis 2023 and 2033, by Molecule
Figure 05: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Molecule
Figure 06: Global Market Attractiveness Analysis 2023 to 2033, by Molecule
Figure 07: Global Market Value Share (%) Analysis 2023 and 2033, by Treatment Type
Figure 08: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Treatment Type
Figure 09: Global Market Attractiveness Analysis 2023 to 2033, by Treatment Type
Figure 10: Global Market Value Share (%) Analysis 2023 and 2033, by Route of Administration
Figure 11: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Route of Administration
Figure 12: Global Market Attractiveness Analysis 2023 to 2033, by Route of Administration
Figure 13: Global Market Value Share (%) Analysis 2023 and 2033, by Distribution Channel
Figure 14: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Distribution Channel
Figure 15: Global Market Attractiveness Analysis 2023 to 2033, by Distribution Channel
Figure 16: Global Market Value Share (%) Analysis 2023 and 2033, by Region
Figure 17: Global Market Y-o-Y Growth (%) Analysis 2022 to 2033, by Region
Figure 18: Global Market Attractiveness Analysis 2023 to 2033, by Region
Figure 19: North America Market Value (US$ Million) Analysis, 2018 to 2022
Figure 20: North America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 21: North America Market Value Share, by Molecule (2023 E)
Figure 22: North America Market Value Share, by Treatment (2023 E)
Figure 23: North America Market Value Share, by Route of Administration (2023 E)
Figure 24: North America Market Value Share, by Distribution Channel (2023 E)
Figure 25: North America Market Value Share, by Country (2023 E)
Figure 26: North America Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 27: North America Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 28: North America Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 29: North America Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 30: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 31: USA Market Value Proportion Analysis, 2022
Figure 32: Global Vs. USA Growth Comparison
Figure 33: USA Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 34: USA Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 35: USA Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 36: USA Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 37: Canada Market Value Proportion Analysis, 2022
Figure 38: Global Vs. Canada. Growth Comparison
Figure 39: Canada Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 40: Canada Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 41: Canada Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 42: Canada Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 43: Latin America Market Value (US$ Million) Analysis, 2018 to 2022
Figure 44: Latin America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 45: Latin America Market Value Share, by Molecule (2023 E)
Figure 46: Latin America Market Value Share, by Treatment (2023 E)
Figure 47: Latin America Market Value Share, by Route of Administration (2023 E)
Figure 48: Latin America Market Value Share, by Distribution Channel (2023 E)
Figure 49: Latin America Market Value Share, by Country (2023 E)
Figure 50: Latin America Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 51: Latin America Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 52: Latin America Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 53: Latin America Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 54: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 55: Mexico Market Value Proportion Analysis, 2022
Figure 56: Global Vs Mexico Growth Comparison
Figure 57: Mexico Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 58: Mexico Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 59: Mexico Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 60: Mexico Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 61: Brazil Market Value Proportion Analysis, 2022
Figure 62: Global Vs. Brazil. Growth Comparison
Figure 63: Brazil Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 64: Brazil Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 65: Brazil Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 66: Brazil Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 67: Argentina Market Value Proportion Analysis, 2022
Figure 68: Global Vs Argentina Growth Comparison
Figure 69: Argentina Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 70: Argentina Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 71: Argentina Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 72: Argentina Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 73: Europe Market Value (US$ Million) Analysis, 2018 to 2022
Figure 74: Europe Market Value (US$ Million) Forecast, 2023 to 2033
Figure 75: Europe Market Value Share, by Molecule (2023 E)
Figure 76: Europe Market Value Share, by Treatment (2023 E)
Figure 77: Europe Market Value Share, by Route of Administration (2023 E)
Figure 78: Europe Market Value Share, by Distribution Channel (2023 E)
Figure 79: Europe Market Value Share, by Country (2023 E)
Figure 80: Europe Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 81: Europe Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 82: Europe Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 83: Europe Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 84: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 85: UK Market Value Proportion Analysis, 2022
Figure 86: Global Vs. UK Growth Comparison
Figure 87: UK Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 88: UK Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 89: UK Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 90: UK Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 91: Germany Market Value Proportion Analysis, 2022
Figure 92: Global Vs. Germany Growth Comparison
Figure 93: Germany Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 94: Germany Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 95: Germany Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 96: Germany Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 97: Italy Market Value Proportion Analysis, 2022
Figure 98: Global Vs. Italy Growth Comparison
Figure 99: Italy Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 100: Italy Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 101: Italy Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 102: Italy Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 103: France Market Value Proportion Analysis, 2022
Figure 104: Global Vs France Growth Comparison
Figure 105: France Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 106: France Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 107: France Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 108: France Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 109: Spain Market Value Proportion Analysis, 2022
Figure 110: Global Vs Spain Growth Comparison
Figure 111: Spain Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 112: Spain Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 113: Spain Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 114: Spain Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 115: Russia Market Value Proportion Analysis, 2022
Figure 116: Global Vs Russia Growth Comparison
Figure 117: Russia Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 118: Russia Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 119: Russia Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 120: Russia Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 121: BENELUX Market Value Proportion Analysis, 2022
Figure 122: Global Vs BENELUX Growth Comparison
Figure 123: BENELUX Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 124: BENELUX Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 125: BENELUX Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 126: BENELUX Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 127: East Asia Market Value (US$ Million) Analysis, 2018 to 2022
Figure 128: East Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 129: East Asia Market Value Share, by Molecule (2023 E)
Figure 130: East Asia Market Value Share, by Treatment (2023 E)
Figure 131: East Asia Market Value Share, by Route of Administration (2023 E)
Figure 132: East Asia Market Value Share, by Distribution Channel (2023 E)
Figure 133: East Asia Market Value Share, by Country (2023 E)
Figure 134: East Asia Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 135: East Asia Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 136: East Asia Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 137: East Asia Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 138: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 139: China Market Value Proportion Analysis, 2022
Figure 140: Global Vs. China Growth Comparison
Figure 141: China Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 142: China Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 143: China Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 144: China Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 145: Japan Market Value Proportion Analysis, 2022
Figure 146: Global Vs. Japan Growth Comparison
Figure 147: Japan Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 148: Japan Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 149: Japan Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 150: Japan Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 151: South Korea Market Value Proportion Analysis, 2022
Figure 152: Global Vs South Korea Growth Comparison
Figure 153: South Korea Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 154: South Korea Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 155: South Korea Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 156: South Korea Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 157: South Asia Market Value (US$ Million) Analysis, 2018 to 2022
Figure 158: South Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 159: South Asia Market Value Share, by Molecule (2023 E)
Figure 160: South Asia Market Value Share, by Treatment (2023 E)
Figure 161: South Asia Market Value Share, by Route of Administration (2023 E)
Figure 162: South Asia Market Value Share, by Distribution Channel (2023 E)
Figure 163: South Asia Market Value Share, by Country (2023 E)
Figure 164: South Asia Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 165: South Asia Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 166: South Asia Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 167: South Asia Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 168: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 169: India Market Value Proportion Analysis, 2022
Figure 170: Global Vs. India Growth Comparison
Figure 171: India Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 172: India Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 173: India Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 174: India Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 175: Indonesia Market Value Proportion Analysis, 2022
Figure 176: Global Vs. Indonesia Growth Comparison
Figure 177: Indonesia Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 178: Indonesia Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 179: Indonesia Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 180: Indonesia Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 181: Malaysia Market Value Proportion Analysis, 2022
Figure 182: Global Vs. Malaysia Growth Comparison
Figure 183: Malaysia Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 184: Malaysia Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 185: Malaysia Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 186: Malaysia Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 187: Thailand Market Value Proportion Analysis, 2022
Figure 188: Global Vs. Thailand Growth Comparison
Figure 189: Thailand Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 190: Thailand Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 191: Thailand Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 192: Thailand Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 193: Oceania Market Value (US$ Million) Analysis, 2018 to 2022
Figure 194: Oceania Market Value (US$ Million) Forecast, 2023 to 2033
Figure 195: Oceania Market Value Share, by Molecule (2023 E)
Figure 196: Oceania Market Value Share, by Treatment (2023 E)
Figure 197: Oceania Market Value Share, by Route of Administration (2023 E)
Figure 198: Oceania Market Value Share, by Distribution Channel (2023 E)
Figure 199: Oceania Market Value Share, by Country (2023 E)
Figure 200: Oceania Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 201: Oceania Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 202: Oceania Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 203: Oceania Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 204: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 205: Australia Market Value Proportion Analysis, 2022
Figure 206: Global Vs. Australia Growth Comparison
Figure 207: Australia Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 208: Australia Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 209: Australia Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 210: Australia Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 211: New Zealand Market Value Proportion Analysis, 2022
Figure 212: Global Vs New Zealand Growth Comparison
Figure 213: New Zealand Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 214: New Zealand Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 215: New Zealand Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 216: New Zealand Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 217: Middle East & Africa Market Value (US$ Million) Analysis, 2018 to 2022
Figure 218: Middle East & Africa Market Value (US$ Million) Forecast, 2023 to 2033
Figure 219: Middle East & Africa Market Value Share, by Molecule (2023 E)
Figure 220: Middle East & Africa Market Value Share, by Treatment (2023 E)
Figure 221: Middle East & Africa Market Value Share, by Route of Administration (2023 E)
Figure 222: Middle East & Africa Market Value Share, by Distribution Channel (2023 E)
Figure 223: Middle East & Africa Market Value Share, by Country (2023 E)
Figure 224: Middle East & Africa Market Attractiveness Analysis by Molecule, 2023 to 2033
Figure 225: Middle East & Africa Market Attractiveness Analysis by Treatment Type, 2023 to 2033
Figure 226: Middle East & Africa Market Attractiveness Analysis by Route of Administration, 2023 to 2033
Figure 227: Middle East & Africa Market Attractiveness Analysis by Distribution Channel, 2023 to 2033
Figure 228: Middle East & Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 229: GCC Countries Market Value Proportion Analysis, 2022
Figure 230: Global Vs GCC Countries Growth Comparison
Figure 231: GCC Countries Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 232: GCC Countries Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 233: GCC Countries Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 234: GCC Countries Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 235: Türkiye Market Value Proportion Analysis, 2022
Figure 236: Global Vs. Türkiye Growth Comparison
Figure 237: Türkiye Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 238: Türkiye Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 239: Türkiye Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 240: Türkiye Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 241: South Africa Market Value Proportion Analysis, 2022
Figure 242: Global Vs. South Africa Growth Comparison
Figure 243: South Africa Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 244: South Africa Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 245: South Africa Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 246: South Africa Market Share Analysis (%) by Distribution Channel, 2023 & 2033
Figure 247: Northern Africa Market Value Proportion Analysis, 2022
Figure 248: Global Vs Northern Africa Growth Comparison
Figure 249: Northern Africa Market Share Analysis (%) by Molecule, 2023 & 2033
Figure 250: Northern Africa Market Share Analysis (%) by Treatment Type, 2023 & 2033
Figure 251: Northern Africa Market Share Analysis (%) by Route of Administration, 2023 & 2033
Figure 252: Northern Africa Market Share Analysis (%) by Distribution Channel, 2023 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
FcRn Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
SGLT2 Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
SGLT2 Inhibitors Treatment Market Overview – Trends & Growth 2024-2034
NF-KB Inhibitors Market
Mould Inhibitors Market
Kinase Inhibitors For Cancer Treatment Market Size and Share Forecast Outlook 2025 to 2035
Enzyme Inhibitors Market
Paraffin Inhibitors Market
Corrosion Inhibitors Market Growth - Trends & Forecast 2025 to 2035
PD-1/PD-L1 Inhibitors Market – Trends, Growth & Forecast 2025 to 2035
Proton Pump Inhibitors Market Insights - Demand, Size & Industry Trends 2025 to 2035
Angiopoietin Inhibitors Therapeutic Market
Small Molecule Inhibitors Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Protein Kinase B Inhibitors Market
Alpha Glucosidase Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
Immune Checkpoint Inhibitors Market
Volatile Corrosion Inhibitors Market Size and Share Forecast Outlook 2025 to 2035
Volatile Corrosion Inhibitors (VCI) Packaging Market Insights - Growth & Demand 2025 to 2035
Low dosage Hydrate Inhibitors Market - Demand, Growth & Industry Outlook 2025 to 2035
Janus Kinase (JAK) Inhibitors Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA