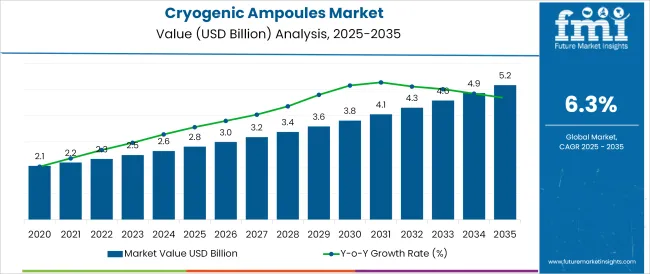
The Cryogenic Ampoules Market is estimated to be valued at USD 2.8 billion in 2025 and is projected to reach USD 5.2 billion by 2035, registering a compound annual growth rate (CAGR) of 6.3% over the forecast period.
The cryogenic ampoules market is experiencing stable growth, supported by advancements in biological storage, increased investment in cold chain infrastructure, and the expanding scope of cell-based therapies and biobanking. Rising pharmaceutical and clinical research activity, particularly in mRNA, stem cell, and immunotherapy fields, is increasing the demand for safe and contamination-resistant primary packaging capable of withstanding cryogenic temperatures.
Adoption is being influenced by stricter regulatory standards for sample integrity and traceability, prompting manufacturers to innovate in ampoule design, material durability, and tamper-proof sealing mechanisms. Growing emphasis on reducing contamination risks and supporting long-term preservation of high-value biologics is fostering the development of sterile, precision-manufactured ampoules.
Future growth will be shaped by increased outsourcing of clinical trials, expansion of cord blood banking, and rapid progress in precision medicine each requiring secure, cryo-stable primary packaging.
The market is segmented by Size and End Use and region. By Size, the market is divided into 1 ml to 5 ml, Up to 1 ml, and Above 5 ml. In terms of End Use, the market is classified into Pharmaceutical Companies, Research Organizations, and Healthcare Institutions.
Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
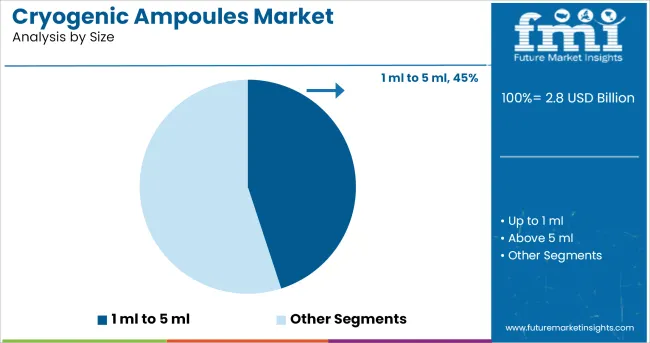
The 1 ml to 5 ml size range is projected to hold 45.0% of the total market revenue in the cryogenic ampoules market in 2025, making it the leading segment. This dominance is being driven by its suitability for handling small-volume, high-value biological samples commonly used in diagnostics, cell therapy, and vaccine storage. Ampoules in this size range offer optimal space efficiency for ultra-low temperature storage systems while minimizing sample wastage.
Their compatibility with automated filling and sealing systems enhances throughput and sterility assurance. Additionally, regulatory compliance and traceability are more easily maintained with smaller ampoule formats, particularly in high-volume clinical and pharmaceutical environments.
As precision dosing and targeted therapies continue to evolve, the 1 ml to 5 ml segment is expected to maintain its leadership, offering balance between usability, safety, and space optimization.
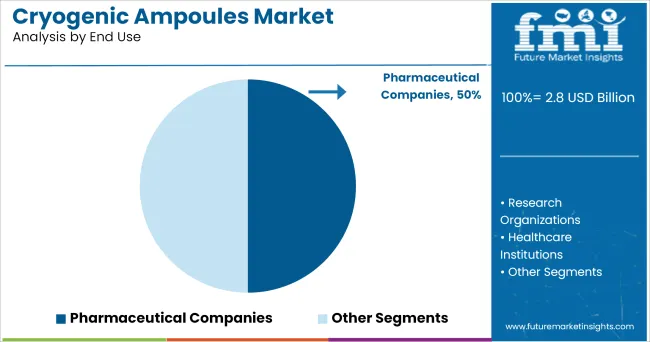
Pharmaceutical companies are projected to account for 50.0% of total market revenue in the cryogenic ampoules market in 2025, making this the dominant end-use segment. Growth in this segment is being driven by increased requirements for cryo-preservation of drug formulations, biologics, and reference standards during R&D and clinical trials.
The need for contamination-free, tamper-evident, and high-integrity containers has reinforced the use of cryogenic ampoules in pharmaceutical environments.
Ampoules provide ideal sterility and batch-level traceability, which are critical for regulatory submissions and quality control. Pharmaceutical firms are also increasingly investing in personalized medicines and cell-based therapies, both of which require secure and consistent cryogenic storage.
With global regulatory bodies tightening standards around biologic storage and shipment, pharmaceutical companies continue to lead demand, leveraging cryogenic ampoules as a reliable primary packaging solution for temperature-sensitive assets.
Recent developments in the pharmaceutical industry and innovative research techniques used in cryogenics require a moisture-proof and safe packaging and storage solution, which is met successfully by cryogenic ampoules.
The ampoules for cryogenics are specially designed and made up of glass to resist materials stored at extremely low temperatures in it, growing number of research organizations and the number of testing done by pharmaceutical companies are also witnessing a sharp rise. Moreover, the global cryogenic ampoules market is expected to grow at a healthy rate in near future.
The increased demand for various types of vaccines and drugs which need to be packaged in a temperature-controlled package and maintained, rise in a number of research and development organizations testing various types of materials and chemicals and their effects.
All these factors result in high demand for cryogenic ampoules, along with the pharmaceutical industry being a major consumer. Thus, the global cryogenic ampoules market is expected to grow positively over the upcoming decade.
Cryogenic ampoules can be only opened by breaking the glass lid of the ampoule, which leads to the risk of small glass particles in the vaccine or chemical packed in it. Also, the cryogenic ampoules are only fit for single use only, these few limitations are likely to hamper the expected growth of the cryogenic ampoules market in coming years.
According to the latest developments by giant pharmaceutical companies and global research, organizations are demanding sustainable product which has no negative effects on the environment after disposing of it. Manufacturers of cryogenic ampoules are offering easy to open ampoules which is easy to store on racks and trays while conducting the test or while transporting the vaccine, it keeps the chemical or drug stored in it safe from moisture, dust and any risk of contamination.
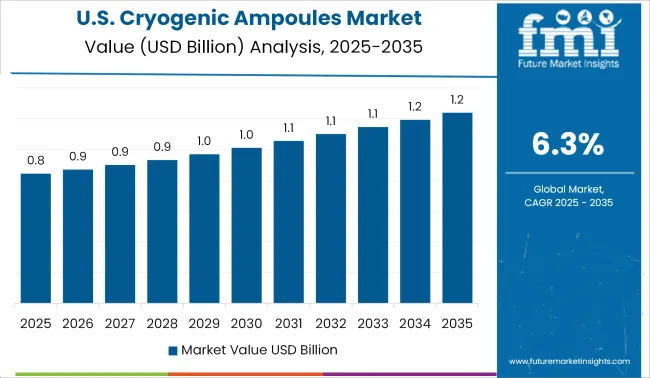
The rise in research and development by the giant pharmaceutical manufacturers and research organizations in the country makes its global hub for research & testing of all kinds of vaccines and chemicals. Also, demand for temperature-controlled packaging is high due to the growing number of research institutes in the country, which makes the USA a major market for the global cryogenic ampoules market.
The growth of a number of medicinal drugs manufacturers in the country and moreover the supply of various expensive chemicals and other fluids which require low-temperature packaging are packed in a cryogenic ampoule, also while transport the risk of damage of chemical is reduced. The entry of domestic manufacturers in the production of cryogenic ampoules indicates the positive impact it has on the growth of the global cryogenic ampoules market.
The easy availability of glass and other raw materials used in manufacturing the cryogenic ampoules and the development of health infrastructure in both countries of India and China, all these factors indicate lucrative growth opportunities offered by these countries.
Also, the ever-growing demand for pharmaceutical drugs and vaccines is driving demand for contamination-free, low-temperature packaging solutions such as cryogenic ampoules in India as well as China. The entry of global key players and the presence of few domestic manufacturers engaged in producing cryogenic ampoules provides a huge untapped market for manufacturers and growth opportunities to the global cryogenic ampoules market for the upcoming decade.
The global cryogenic ampoules market is estimated to be valued at USD 2.8 billion in 2025.
The market size for the cryogenic ampoules market is projected to reach USD 5.2 billion by 2035.
The cryogenic ampoules market is expected to grow at a 6.3% CAGR between 2025 and 2035.
The key product types in cryogenic ampoules market are 1 ml to 5 ml, up to 1 ml and above 5 ml.
In terms of end use, pharmaceutical companies segment to command 50.0% share in the cryogenic ampoules market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Analyzing Cryogenic Ampoules Market Share & Industry Leaders
Cryogenic Helium Cycling System Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Vial Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Label Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Temperature Controller Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Vaporizer Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Air Separation Unit Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Freezers Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Systems Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Boxes Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Tanks Market Size and Share Forecast Outlook 2025 to 2035
Ampoules Packaging Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Capsules Market Growth - Demand & Forecast 2025 to 2035
Cryogenic Vials and Tubes Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Pump Market Size & Trends 2025 to 2035
Cryogenic Valves Market Growth - Trends & Forecast 2025 to 2035
Competitive Overview of Cryogenic Insulation Films Companies
Market Share Distribution Among Cryogenic Label Providers
Cryogenic Insulation Films Market Report – Demand, Trends & Industry Forecast 2025-2035
Cryogenic Technology Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA