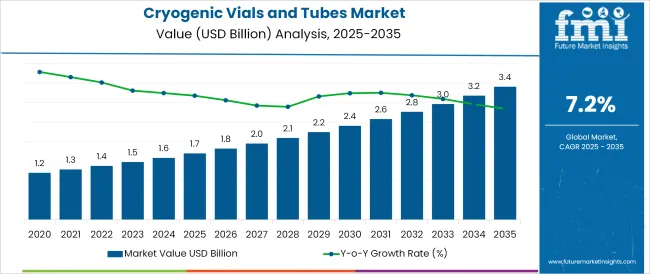
The Cryogenic Vials and Tubes Market is estimated to be valued at USD 1.7 billion in 2025 and is projected to reach USD 3.4 billion by 2035, registering a compound annual growth rate (CAGR) of 7.2% over the forecast period.
The cryogenic vials and tubes market is experiencing sustained growth as bio banking, clinical research, and pharmaceutical manufacturing expand globally. Demand for secure, contamination free, and efficient sample storage solutions at ultra-low temperatures has been intensifying, supported by increased investment in genomics, cell therapy, and vaccine development.
The market is being shaped by rising adoption of automated cry storage systems and the need to comply with stringent biosafety and quality standards. Technological innovations in vial and tube materials, coupled with improved sealing and traceability features, are enhancing usability and reliability in critical applications.
Future growth is expected to benefit from the expansion of biopharmaceutical pipelines, government initiatives supporting life sciences infrastructure, and the growing focus on personalized medicine. Improvements in material integrity and ergonomic design are paving the way for broader adoption across research and clinical settings.
The market is segmented by Material Type, Product, Thread, and Capacity and region. By Material Type, the market is divided into Plastic and Glass. In terms of Product, the market is classified into Round bottom vials and tubes, Shaped bottom vials and tubes, and Bracket bottom vials and tubes. Based on Thread, the market is segmented into External vial thread and Internal vial thread.
By Capacity, the market is divided into 1.0-5 ml, 0.2-0.5 ml, and 0.5-1.0 ml. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
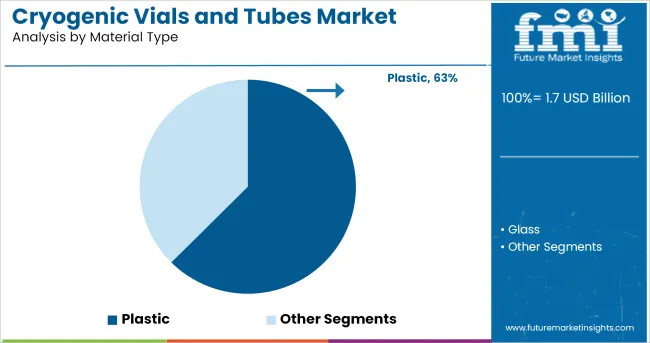
When segmented by material type, plastic is expected to hold 62.5% of the total market revenue in 2025, maintaining its position as the dominant material. This leadership is attributed to the superior durability, lightweight nature, and cost effectiveness of advanced plastic polymers used in cryogenic conditions.
The ability of high-grade plastics to withstand extreme low temperatures without compromising structural integrity has positioned them as the preferred choice over alternatives. Enhanced chemical resistance and compatibility with automation systems have further strengthened their suitability for bio banking and laboratory workflows.
Manufacturers have optimized plastic formulations to minimize leachable and maintain sample purity, which has driven adoption in regulated environments. These advantages have ensured that plastic remains the material of choice for both high volume and specialized cryogenic storage applications.
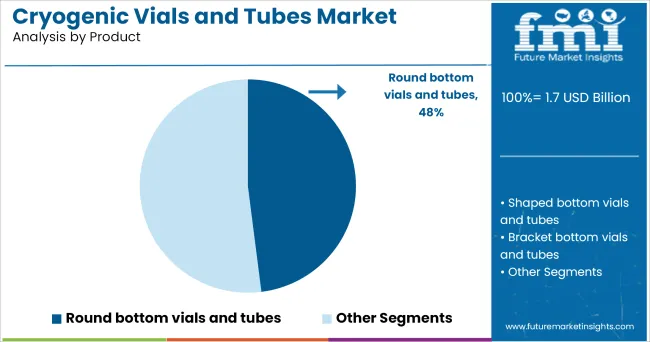
In terms of product type, round bottom vials and tubes are projected to account for 48.0% of the market revenue in 2025, emerging as the leading product segment. This segment’s prominence has been driven by the ergonomic advantages and ease of use offered by the round bottom design, which facilitates complete sample retrieval and efficient centrifugation.
The smooth contours minimize residue retention and improve mixing performance, meeting the practical demands of laboratory technicians handling sensitive biological materials. The design also supports better distribution of thermal stress during freezing and thawing cycles, which contributes to enhanced durability.
Adoption has been reinforced by their compatibility with a wide range of racks and automated storage systems, making them versatile for both research and clinical applications. These functional benefits have consistently positioned round bottom vials and tubes at the forefront of user preference.
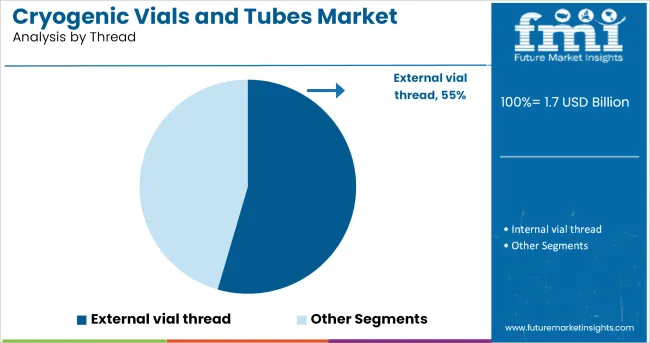
When segmented by thread type, external vial thread is anticipated to capture 54.5% of the market revenue in 2025, establishing itself as the leading thread configuration. This dominance has been supported by the superior sealing performance and reduced contamination risk provided by external threading, which prevents contact between the sample and the thread itself.
Laboratory users have favored this design for its ability to maintain sterility and prevent sample loss during storage and handling. The ergonomic advantage of smoother opening and closing motions without compromising the integrity of the seal has further strengthened its adoption.
External threads also minimize the accumulation of ice and contaminants, making them more reliable in cryogenic conditions. These combined benefits have secured the external vial thread segment’s leadership as the preferred choice in environments where sample integrity and operator efficiency are critical.
Cryogenic vials and tubes are the types of pharmaceutical packaging containers which are used to store biological samples at extreme low temperature, which can be as low as -196° C. The biological samples are stored or preserved with the help of liquid nitrogen, which can stand extreme temperatures without compromising the samples.
Cryogenic vials and tubes are constructed from non-mutagenic plastic resins, non-cytotoxic plastics and therefore are pyrogen free. Majority of the cryogenic vials and tubes have screw-style closure which helps the vials and tubes to stand extreme temperature and air pressure. Cryogenic vials and tubes have either external or internal threads which helps to maintain the concentration of the sample.
Cryogenic tubes are also provided with the writing and marking area which can be used to identify the sample. Colour coding markers or caps on the cryogenic tubes are also used to identify the sample.
The automated processing and dispensing of the multiple vials in parallel instead of manual processing and dispensing is increasing the quality and consistency of the cryogenic vials and tubes and also avoids occupational injuries.
The alternative vial dispensing systems which makes the use of peristaltic pumps to fill the vials which reduces the shear damage and regulates viability before and after freezing.
Therefore, the automated cryovial processing equipment not only decreases the manual work but also increases the quality of the biological sample packing in the cryogenic vials.
Cryogenic vials and tubes are used to store the biological samples in the extreme lower temperature for the longer time. Centrifugal tubes cannot be used to store the sample for the longer time as they have the risk of breaking the vial or the tube.
Also, the cryogenic vials and tubes provide better stability to the biological sample especially RNA samples owing to the tight closure of the vial and stabilized storage of sample in the extreme low temperatures.
The tight closure of the vial or the tube restricts the evaporation of the biological sample from the container, which is mostly preferred over the centrifugal tubes. Unlike the centrifugal tubes, the cryogenic vials does not need to autoclave for the sterilization.
Cryogenic vials can be sterilized using the gamma radiations which are then packed in the tamper-proof and re-sealable safety-lock bags.
The manufacturing of the cryogenic vials and tubes is a very sensitive process and needs the extra sensitive handling while storing the biological sample. The improper filling of liquid nitrogen gas can lead to generate the pressure during thawing in the cryogenic vial which can then lead to change the biological properties of the sample or may destroy the sample.
Also, if the liquid phase storage is necessary, the cryogenic heat shrink tubing is required to encase the cryogenic vial.
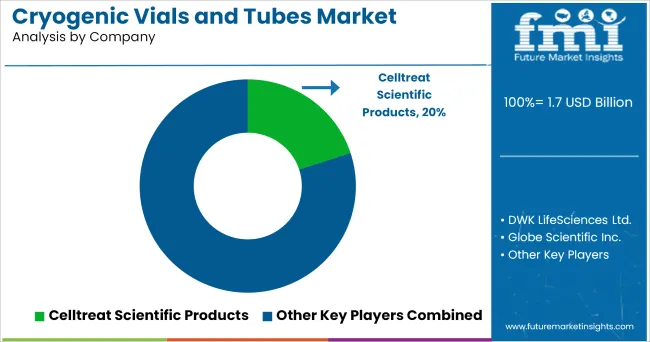
The following global key players such as
Key Asian players manufacturing cryogenic vials and tubes are
The manufacturers involved in the expansion of the production facilities owing to the increased demand for the cryogenic vials and tubes. Also, some key manufacturers in the market are focused on the acquisitions of the small market players operating in the cryogenic vials and tubes in order to increase their presence and to enhance product development.
The increasing rapid development of the healthcare facilities such as, hospitals and pharmaceutical companies in India is the factor bolstering the growth of the cryogenic vials and tubes market. Also, the presence of large number of small entrants and the key players involved in manufacturing of the cryogenic vials and tubes is a responsible factor soaring the growth of the cryogenic vials and tubes market.
Adding to, also the changing technologies and the innovations by emphasizing on research & development activities in India is acting as a boon towards the growth of the cryogenic vials and tubes market.
The global cryogenic vials and tubes market is estimated to be valued at USD 1.7 billion in 2025.
The market size for the cryogenic vials and tubes market is projected to reach USD 3.4 billion by 2035.
The cryogenic vials and tubes market is expected to grow at a 7.2% CAGR between 2025 and 2035.
The key product types in cryogenic vials and tubes market are plastic and glass.
In terms of product, round bottom vials and tubes segment to command 48.0% share in the cryogenic vials and tubes market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Cryogenic Vial Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Label Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Temperature Controller Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Vaporizer Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Air Separation Unit Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Freezers Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Systems Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Boxes Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Tanks Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Ampoules Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Capsules Market Growth - Demand & Forecast 2025 to 2035
Cryogenic Pump Market Size & Trends 2025 to 2035
Cryogenic Valves Market Growth - Trends & Forecast 2025 to 2035
Competitive Overview of Cryogenic Insulation Films Companies
Market Share Distribution Among Cryogenic Label Providers
Cryogenic Insulation Films Market Report – Demand, Trends & Industry Forecast 2025-2035
Analyzing Cryogenic Ampoules Market Share & Industry Leaders
Cryogenic Technology Market
Cryogenic vial rack Market
Non-Cryogenic Air Separation Unit Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA