The Cytotoxicity Assays Market is estimated to be valued at USD 18.0 billion in 2025 and is projected to reach USD 43.1 billion by 2035, registering a compound annual growth rate (CAGR) of 9.1% over the forecast period.
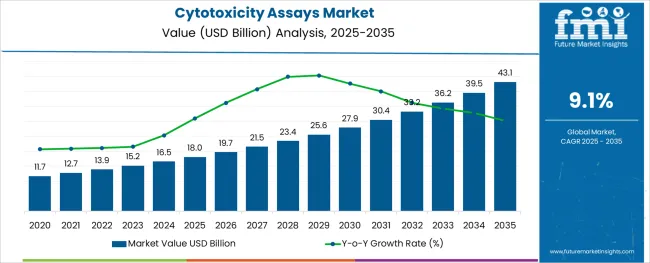
The cytotoxicity assays market is expanding due to the increasing need for evaluating the safety and efficacy of pharmaceutical compounds and chemicals. The rise in drug discovery activities, particularly in early-stage toxicity screening, has driven demand for reliable and sensitive assay methods. Advancements in assay technologies have enabled more precise and reproducible detection of toxic effects on cells, supporting better decision-making in research and development.
The growing prevalence of chronic diseases and the demand for personalized medicine have further encouraged adoption in clinical and preclinical research settings. Regulatory requirements for safety testing have also reinforced the use of cytotoxicity assays across industries.
As pharmaceutical pipelines grow and novel therapies are developed, the market is expected to advance with ongoing innovations in assay kits and protocols. Segment growth is expected to be led by cytotoxicity assay-based kits as the preferred product type, nephrotoxicity as a key application, and hospitals as major end users.
The market is segmented by Product Type, Application, and User Type and region. By Product Type, the market is divided into Cytotoxicity Assay Based Kits, Colorimetric Cytotoxicity Based Assays Kits, Fluorometric Cytotoxicity Based Assays Kits, Elisa Kits, Crystal Violet Kits, and Minimal Inhibitory Concentration Cytotoxicity Assay Kits. In terms of Application, the market is classified into for Nephrotoxicity, for Cardiotoxicity, for Apoptosis, for Cell Proliferation, for Immunohistochemistry, for Reporter Genes, for Transfection Efficacy, and for Other Applications. Based on User Type, the market is segmented into for Hospitals, for Diagnostics Centers, for Academic & Research Laboratories, for Pharma & Biotech Companies, and for Other End User Types. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
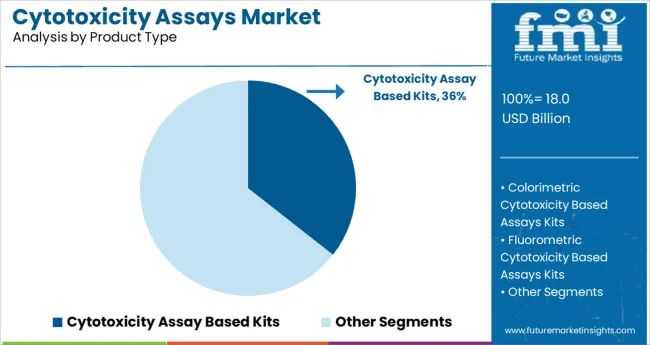
The cytotoxicity assay-based kits segment is projected to hold 35.6% of the market revenue in 2025, leading product type adoption. These kits offer standardized, ready-to-use solutions that simplify the process of measuring cell viability and toxicity. Their convenience, accuracy, and compatibility with high-throughput screening platforms have made them highly popular in research laboratories.
Continuous innovation in assay sensitivity and multiplexing capabilities has further boosted their utilization. The ability to assess a range of cell types and toxic effects makes these kits versatile tools for early-stage drug safety evaluation.
Additionally, ease of use and reproducibility have enhanced their appeal in clinical research environments. The segment is expected to maintain growth as demand for rapid and reliable cytotoxicity testing increases.
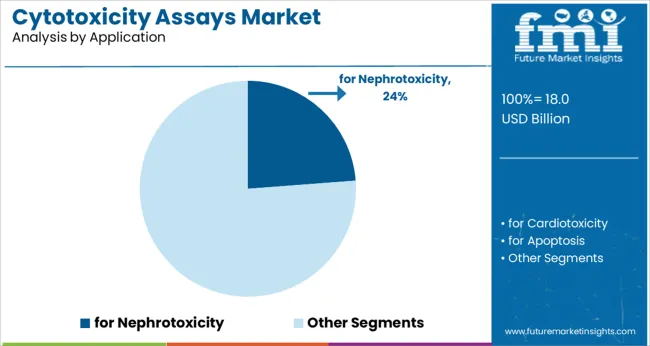
The nephrotoxicity application segment is anticipated to capture 23.8% of the market revenue in 2025, marking it as a significant focus area. Growing concerns over drug-induced kidney damage have highlighted the need for specific toxicity assays to assess nephrotoxic effects early in drug development.
Regulatory guidance has emphasized kidney safety profiling, driving adoption of targeted cytotoxicity assays in pharmaceutical research. As kidney-related adverse effects remain a common cause of drug attrition and clinical complications, nephrotoxicity testing has become an integral part of safety evaluations.
Advances in cell-based models mimicking kidney tissue have enhanced assay relevance and predictive accuracy. The segment’s importance is reinforced by the rising incidence of kidney diseases worldwide and increasing investments in nephrotoxicity research.
The hospitals segment is projected to account for 29.4% of the cytotoxicity assays market revenue in 2025, maintaining its leading position among user types. Hospitals have increasingly adopted cytotoxicity assays for clinical diagnostics and monitoring, particularly in oncology and toxicology departments.
The need for personalized treatment regimens and monitoring of chemotherapy toxicity has driven demand for reliable cytotoxicity testing in hospital laboratories. Integration of cytotoxicity assays with patient care workflows allows for better assessment of drug safety and treatment efficacy.
Additionally, hospital-based research and diagnostic facilities have expanded their capabilities in molecular and cellular testing, boosting assay utilization. With the growing emphasis on precision medicine and patient safety, the hospitals segment is expected to sustain its significant role in the cytotoxicity assays market.
One of the most important indicators used in the biological evaluation system is the cytotoxicity assay. Many experimental methods for evaluating cell cytotoxicity are constantly being developed and modernized as modern cell biology advances.
Furthermore, some manufacturers have developed real-time and dynamic methods for quantifying cell morphology, proliferation, and differentiation in cytotoxicity assays.
Following this, other players are attempting to incorporate new real-time cell analysis system technologies into in vitro cytotoxicity tests of medical devices in order to meet the objectives of various tests and also to evaluate the accuracy of in vitro cytotoxicity assays.
One significant disadvantage is the lack of uniform cytotoxicity test methods. Aside from that, there is no standardized protocol for performing cytotoxicity assays.
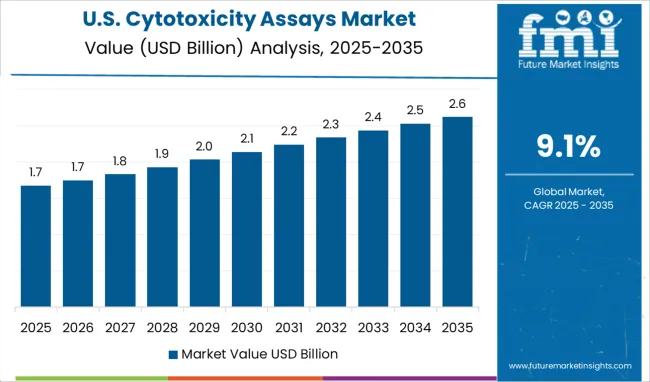
During the forecast period, North America is expected to hold one of the largest shares of the global cytotoxicity assays market. The region's drivers include an increase in haematology and cell proliferation studies, oncology studies, and a variety of other cell-based studies.
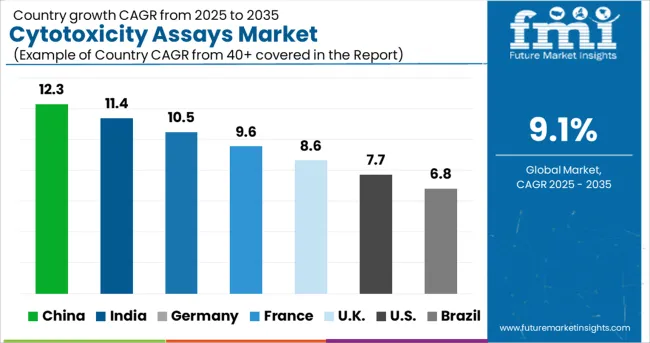
According to Future Market Insights, Asia-Pacific countries, particularly India and China, are expected to grow at a significant rate over the assessment period.
Factors such as a significant increase in the import of these assay kits from leading global manufacturers, as well as the presence of local manufacturers with R&D facilities in the region will drive the growth of the cytotoxicity assays market in this region. Japan, with its high R&D investments in medical devices and well-established healthcare infrastructure, will pave the way for the country's cytotoxicity assay market to grow.
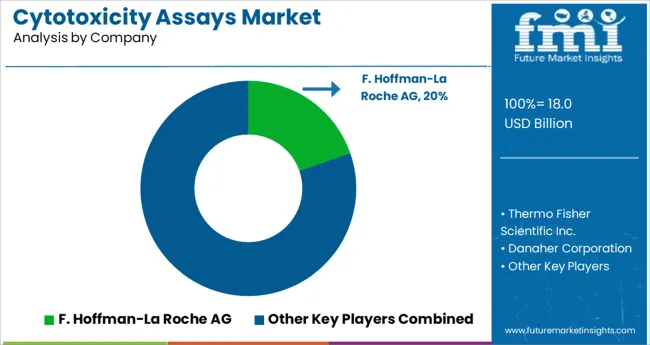
Some of the key participants present in the global Cytotoxicity Assays market include F. Hoffman-La Roche AG Thermo Fisher Scientific, Inc, Danaher Corporation, Sakura Finetechnical Co., Ltd and Laboratory Corporation of America Holdings Merck KGaA among others.
Attributed to the presence of such high number of participants, the market is highly competitive.
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 9.1% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in billion, Volume in Kilotons and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered | Product Type, Application, End User, Region |
| Regions Covered | North America; Latin America; Europe; South Asia; East Asia; Oceania; Middle East & Africa |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, Nordics, BENELUX, Russia, Australia & New Zealand, China, Japan, South Korea, India, Thailand, Malaysia, Indonesia, GCC, South Africa |
| Key Companies Profiled | F. Hoffman-La Roche AG; Thermo Fisher Scientific Inc.; Danaher Corporation; Sakura Finetechnical Co., Ltd; Laboratory Corporation of America Holdings; Merck KGaA |
| Customization | Available Upon Request |
The global cytotoxicity assays market is estimated to be valued at USD 18.0 billion in 2025.
It is projected to reach USD 43.1 billion by 2035.
The market is expected to grow at a 9.1% CAGR between 2025 and 2035.
The key product types are cytotoxicity assay based kits, colorimetric cytotoxicity based assays kits, fluorometric cytotoxicity based assays kits, elisa kits, crystal violet kits and minimal inhibitory concentration cytotoxicity assay kits.
for nephrotoxicity segment is expected to dominate with a 23.8% industry share in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Custom Assays Market
Diabetic Assays Market
Angiogenesis Assays Market Insights – Growth & Forecast 2025 to 2035
Lateral Flow Assays Market Analysis - Size, Share & Forecast 2025 to 2035
Multiplex PCR Assays Market Size and Share Forecast Outlook 2025 to 2035
Mycology Immunoassays Testing Market Size and Share Forecast Outlook 2025 to 2035
Dual Biomarker Assays Market Analysis - Size, Share, & Forecast Outlook 2025 to 2035
Organ Function Assays Market
Market Share Insights of Leading Protein Binding Assays Providers
Transport Protein Assays Kits Market Trends - Industry Forecast 2025 to 2035
Biomarker-based Immunoassays Market Size and Share Forecast Outlook 2025 to 2035
Fibrin Degradation Product (FnDP) Assays Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA