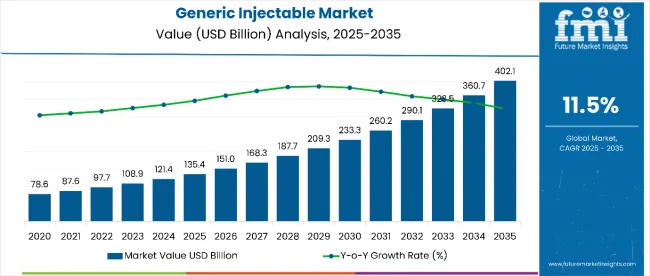
The global sales of generic injectable are estimated to be worth USD 135.4 billion in 2025 and are anticipated to reach a value of USD 402.1 billion by 2035. Sales are projected to rise at a CAGR of 11.5% over the forecast period between 2025 and 2035. The revenue generated by generic injectable in 2024 was USD 120.7 billion.
The number of FDA approvals for the new generic entity is experiencing a great deal of surge in the generic pharmaceutical industry, primarily due to several factors such as increasing number of patent expiries, government policy and demographics, new higher-value opportunities in complex generics and biosimilars, pace of abbreviated new drug application (ANDA) approvals by FDA, and introduction of cost-effective injectable through mergers and consolidation in the generic injectable market.
The key factors in the growth of the generic injectable market include the nature and degree of portfolio differentiation, as well as the scale and age of manufacturing facilities. Increasing incidence of chronic diseases particularly cancers, cardiovascular diseases, diabetes and others across the globe have been the prime drivers for this revenue sales.
While cancers witness frequent drug shortages where the market access to such medicines is limited by very few players. There has been large increases in production of insulin in order to contain the prevalence of diabetes, most noted in emerging countries.
Global Generic Injectable Industry Analysis
| Attributes | Key Insights |
|---|---|
| Historical Size, 2024 | USD 120.7 billion |
| Estimated Size, 2025 | USD 135.4 billion |
| Projected Size, 2035 | USD 402.1 billion |
| Value-based CAGR (2025 to 2035) | 11.5% |
The generic injectables market has been witnessing significant growth, driven by factors such as increased investments in manufacturing facilities and favorable government policies. In July 2020, Pfizer announced an approximate investment of USD 465.0 million in a state-of-the-art sterile injectable plant in Portage, Michigan.
This kind of investment by big pharma players shows how better infrastructure could enable generic injectables production. Sterile product manufacturing capability is fundamental to the development and supply of the injectable medications that play critical roles as therapies within many lifesaving disease categories, which also include cancers, cardiovascular diseases, and acute infections.
Apart from these, tax reforms and government incentives have given a very encouraging economic climate to firms for localization of production and investing in advanced manufacturing technologies.
This has enabled not only a rise in the capacity level of meeting the emerging global demand but also brought in supply chain resilience by reducing dependence on international suppliers.
Investments like Pfizer's are indicative of the broader trend in the generic injectables market, where continued sterile manufacturing innovation, in conjunction with regulatory support, furthers growth and increases access to life-sustaining medicines across the globe.
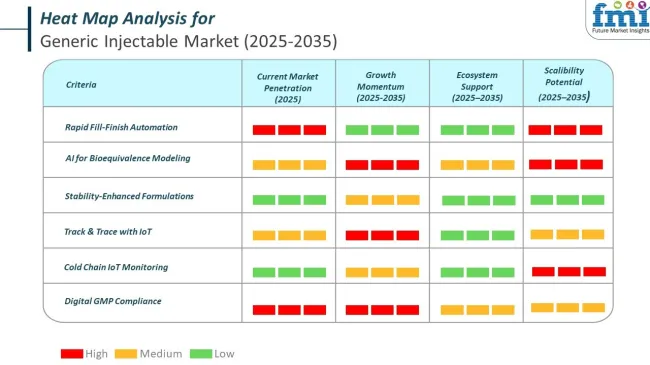
Government oversight plays a critical role in shaping the supply, safety, and affordability of generic injectables. Regulatory bodies focus on ensuring bioequivalence, maintaining GMP (Good Manufacturing Practice) compliance, and accelerating approvals while safeguarding patient safety. As global demand rises, harmonization efforts and inspection protocols are evolving to keep pace with innovation, cost pressures, and complex biologics.
In the generic injectable market, top companies are actively embedding AI, automation, and smart systems into operations. These changes aren’t just cost-saving, they redefine quality, speed, and compliance in ways that are shaping 2025’s industrial landscape. Sales cycles are compressing and demand is shifting toward digitally optimized players.
A comparative analysis of fluctuations in compound annual growth rate (CAGR) for the generic injectable industry outlook between 2024 and 2025 on a six-month basis is shown below.
By this examination, major variations in the performance of these markets are brought to light, and trends of revenue generation are captured hence offering stakeholders useful ideas on how to carry on with the market’s growth path in any other given year. January through June covers the first part of the year called half1 (H1), while half2 (H2) represents July to December.
The table below compares the compound annual growth rate (CAGR) for the global generic injectable market from 2024 to 2025 during the first half of the year. This overview highlights key changes and trends in revenue growth, offering valuable insights into market dynamics.
H1 covers January to June, while H2 spans July to December. In the first half (H1) of the decade from 2024 to 2034, the business is predicted to surge at a CAGR of 5.2%, followed by a slightly lower growth rate of 4.9% in the second half (H2) of the same decade.
| Particular | Value CAGR |
|---|---|
| H1 (2024 to 2034) | 5.2% |
| H2 (2024 to 2034) | 4.9% |
| H1 (2025 to 2035) | 11.5% |
| H2 (2025 to 2035) | 4.0% |
Moving into the subsequent period, from H1 2025 to H2 2035, the CAGR is projected to decrease slightly to 11.5% in the first half and projected to lower at 4.0% in the second half. In the first half (H1) the market witnessed a decrease of 70 BPS while in the second half (H2), the market witnessed a decrease of 90 BPS.
Product Innovations and Regulatory Approvals Fueling Growth in the Generic Injectables Market
Product innovations and regulatory approvals have driven the generic injectables market, which have been the key in facilitating access to affordable healthcare. A case in point is that of Hikma Pharmaceuticals PLC, which recently received FDA approval for its generic version of Victoza®.
This makes for the clearest indication that innovation within the injectables market is most definitely not standing still and the generic version of a complex biologic is available as a cost-effective alternative. The GLP-1 product liraglutide improves glycemic control in patients with type 2 diabetes and underlines the potential to address critical medical needs with generic injectables at a fraction of the cost.
The improvement of technologies in manufacturing, regulatory frameworks, and the expiry of branded drugs' the market is being driven in few years. With firms in the pharmaceutical industry continuing to get approval for new generics, they are now well-positioned to offer effective and low-cost alternatives to expensive brand-name medications as needed in the growing demand for budget-friendly options.
The FDA approval of generics for complex injectable products underscores the regulatory confidence in their safety and efficiency, which ultimately adds to market growth.
Thus, the continuous advancements in manufacturing, regulatory approvals, and the introduction of generics for complex injectables are collectively fueling the rapid growth of the generic injectables market.
Frequent Drug Shortages Boosting the Growth of the Generic Injectable Market
Frequent drug shortages in the healthcare industry have now been conclusively acknowledged as a major driving force behind generic injectable market growth.
These shortages are the result of disruptions in the supply chain, manufacturing delays, and heightened demand-for reliable and affordable alternatives. Generic injectables, essentially bioequivalent to their branded counterparts, offer a timely solution since they ensure a constant supply of these essential drugs.
While generic injectables are rapidly gaining popularity, they are being supplied to meet various niche demands in very core areas such as pain management, diabetes, and oncology.
Compared to new medications, generic injectables yield lower prices and shorter cycles of development for the manufacturer. In this sense, the adverse effects of drug shortages on continued healthcare delivery will only multiply until the innovative efforts in this area produce viable alternatives through nonproprietary options.
While the prevalence of drug shortages in healthcare delivery continues, the search for viable alternatives has become one of increased urgency; hence, generic injectables are important in ensuring the continuous supply of important medicines toward global healthcare needs.
FDA Research Highlights Opportunities in the Generic Injectable Market
The FDA’s commitment to enhancing public understanding and awareness of generic drugs presents significant opportunities for the growth of the generic injectable market. By programs such as the Science and Research program under the Genric Drug User Fee Amendments (GDUFA), the FDA provides scientific scrutiny to generic drug applications to ensure that they are safe, effective, and of adequate quality.
An example of this is a qualitative study recently published by the FDA and involving patients with chronic obstructive pulmonary disease (COPD) or asthma. These participants, who were actively using brand-name dry powder inhalers, shared their perceptions of generic drug substitution.
The study revealed valuable insights into patient attitudes toward the quality, efficacy, design, and usability of generic products. Such research informs educational initiatives aimed at addressing misconceptions and building trust in generics.
This emphasis on understanding patient perspectives extends to other critical drug categories, including injectables. Healthcare providers and patients stand to benefit greatly from increased confidence in the quality, safety, and efficacy of generic injectable products; FDA's proactive research and engagement are catalysts for market growth and reveal the previously untapped potential of generic injectables in meeting unmet medical need and lowering the burden of health care costs for patients and systems around the world.
Restraining Factors Impacting the Growth of the Generic Injectable Market
While the generic injectable market has immense potential, several restraining factors can hinder the growth. A major hurdle has been the complex and costly manufacturing process associated with injectables.
In contrast to oral generics, injectables have more stringent quality control and involve specialized equipment to be manufactured in strictly regulated environments, which makes the production development process costlier and lengthier.
Higher manufacturing costs for injectables are typically disadvantageous for small-scale manufacturers in the market, thus slowing competition for cheap generic injectables.
Further, these include limited exclusivity for some generic injectables, with complex biologics or high-tech formulations. Most biosimilars are a generic form of biologic drugs and require substantially enhanced technology coupled with scientific expertise, which can be a stumbling block for many manufacturers. Furthermore, regulatory hurdles during approval and the requirement for vast clinical data to prove bioequivalence is another significant delay for market entry.
Intellectual property issues, including patent disputes and exclusivity rights granted to branded drugs, could legitimately serve as bottlenecks for generic injectable producers. Such legal hurdles could extend the presence period of the branded products and shut the door to generics.
The global generic injectable industry recorded a CAGR of 12.7% during the historical period between 2020 and 2024. The growth of the generic injectable diagnostics industry was positive as it reached a value of USD 120.7 billion in 2024 from USD 74.7 billion in 2020.
In the past few years, the generic injectable market has significantly evolved, with various trends affecting its shape. It had been relatively underdeveloped due to the complex and costly nature surrounding the manufacture of injectables compared with oral generics.
The increased availability of branded injectable products expiring on patent, especially for biologics, however, sparked a steady interest in the generic injectable space. 2015 marked the first pivotal milestone in that evolution as the very first generic biologic was approved for use; from that point on, biosimilars began their life as complex generics in all but name.
Looking ahead, the generic injectable market is ready to undertake steady growth. The number of biologics losing patent protection will drive the growth of the biosimilar and generic injectable markets.
Improvements in drug delivery systems, including prefilled syringes and autoinjectors, will work towards improved usability and accelerate uptake in areas such as oncology, diabetes management, and pain control.
Furthermore, the ever-expanding focus on personalized medicine will propel the advent of highly specialized generics. With these trends, the market is on the verge of growth as more affordable and high-quality injectables come to the patients around the globe.
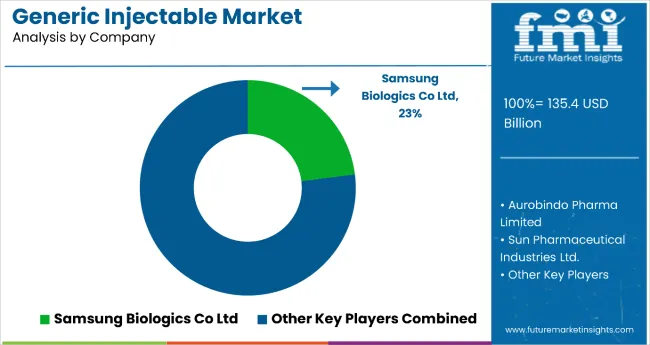
Tier 1 players in this market holds 58.0%. In the tier 1 are companies with a significant global presence, such as Samsung Biologics Co Ltd , Aurobindo Pharma Limited, Sun Pharmaceutical Industries Ltd., and Novartis AG, Merck & .
Top companies lead the generic injectable field. They have a wide range of injectable drugs, from simple types to complex biosimilars. They work closely with regulators, have big factories, and enjoy wide-reaching supply networks worldwide.
In the tier 2, companies like Cipla Ltd, Pfizer Inc, Fresenius Kabi, Sanofi S.A, AstraZeneca Plc are prominent players that occupies around 33.0% market share with a solid presence in specific regional markets.
Tier 2 companies often look at new markets where there's less fierce competition. They meet the need for cheaper branded injectables. These companies are often quicker to react to changing demands and can launch products faster than big firms.
The section below covers the generic injectable industry analysis for the sales for different countries. Market demand analysis on key countries in several regions of the globe, including North America, Latin America, East Asia, South Asia & Pacific, Western Europe, Eastern Europe, and Middle East & Africa is provided.
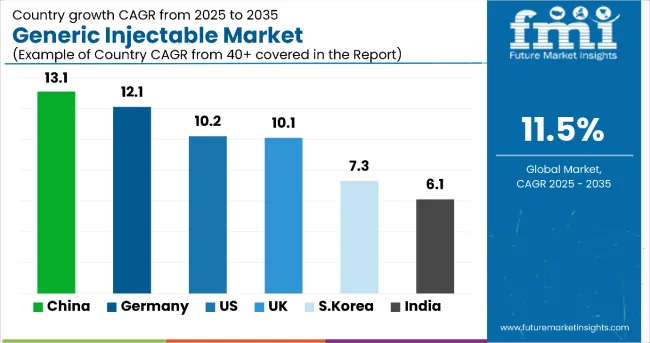
The United States is anticipated to remain at the forefront in North America, with a CAGR of 10.2% through 2035. In South Asia & Pacific, India is projected to witness the highest CAGR in the market of 12.1% by 2035.
| Countries | Value CAGR (2025 to 2035) |
|---|---|
| United States | 10.2% |
| Germany | 12.1% |
| UK | 10.1% |
| China | 13.1% |
| India | 6.1% |
| South Korea | 7.3% |
The United States market dominates the global market with high share in 2024. The United States is expected to exhibit a CAGR of 10.2% throughout the forecast period (2025 to 2035).
The United States has emerged as a leader in the generic injectable market due to the conducive regulatory environment and size of the healthcare market.
For approval of generic injectables, the USA FDA has worked out certain regulatory mechanisms facilitating faster approvals, especially in the case of higher burden of diseases, such as cancer and diabetes. Each of these improved devices lessens the time it takes for generics to enter the market, thus making patients have the cheaper options for medicines.
The immense size of the USA healthcare system-linked to overcoming the growing pharmaceutical costs through generics-probes further augments the demand for generic injectables. Generic alternative quickly fills the empty steads as several branded injectables patents expire, average these majorly being in oncology, autoimmune diseases, and critical care.
Take into consideration the pressure of major pharmaceutical companies and competitive market environment, making availability even better amongst these generic products; USA is the largest market for generic injectables across the globe.
In 2024, Germany market held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 12.1%.
The European generic injectable landscape is dominated much by Germany because of its diverse market of biosimilars. The presence of elaborate pharmaceutical infrastructure and compliance with regulations from the European Union ensures that the generics are manufactured within the required quality standards, making them ideal in different health systems across Europe.
Furthermore, Eu’s strategic plan for the manufacturing of generic injectables in Germany, it is making easy to access other neighboring markets, thus aiding in the exportation of generic injectables.
The well-structured health reimbursement plan in Germany is also acting as a facilitator for ensuring that generics find their way into the market which, in turn, boosts the investments in readying the production models of injectable biosimilars. Therefore, as branded biologics patent brownouts in Germany, its pharmaceutical industry is carrying on leading in the supply of cost-efficient generic injectable solutions.
India occupies a leading value share in South Asia & Pacific market in 2024 and is expected to grow with a CAGR of 6.1% during the forecasted period.
Indian market remains a remarkable player in the global generic injectable market with its capacity of producing high-quality injectables at a fraction of the cost of branded drugs.
Low manufacturing costs caused by the availability of a large pool of skilled talents allow Indian companies to produce sterile injectables at much lower prices. This cost factor is the major advantage India possesses to stand as a strong supplier of generic injectables in both emerging and mature markets.
The Indian pharmaceutical giants like Dr. Reddy's Laboratories, Sun Pharma, and Cipla are already making significant inroads into the markets of the USA and Europe, where the demand for affordable generics is increasing. Focus on scaling up the production of both simple and complex injectables, including biosimilars, meets a growing worldwide demand for affordable medications.
In addition, the detailed regulatory framework and the effective Central Drugs Standard Control Organization (CDSCO) ensure compliance with international standards of Indian generics that hugely boost their global image and acceptance.
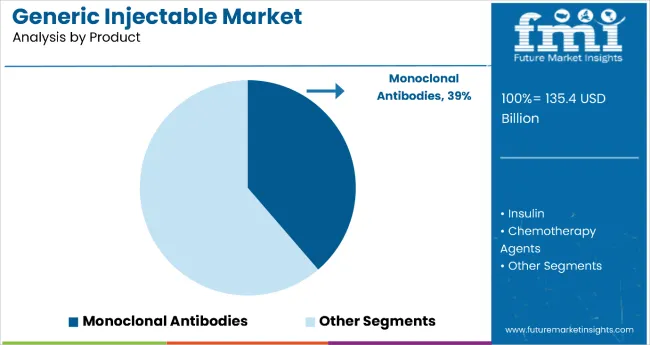
Monoclonal antibodies hold the dominant position with 38.7% of the market share in the product category within the generic injectable market. This leadership is driven by monoclonal antibodies' exceptional therapeutic versatility and effectiveness across oncology, autoimmune diseases, and chronic conditions. The extensive application of mAbs in treating various cancers and their development of generic versions following patent expirations of blockbuster biologics has solidified their market dominance, providing cost-effective alternatives to branded counterparts.
The segment's dominance is reinforced by the substantial demand for monoclonal antibodies driven by their targeted mechanism of action and superior efficacy profiles compared to traditional therapies. The approval of generic versions through biosimilar pathways, supported by regulatory frameworks like the FDA Biosimilars Action Plan, has accelerated market entry and clinical adoption. As the development process continues to identify specific antigens for targeting and healthcare systems increasingly adopt biologic treatments, the monoclonal antibodies segment is positioned to maintain its market leadership through continued therapeutic innovation and expanded access initiatives.
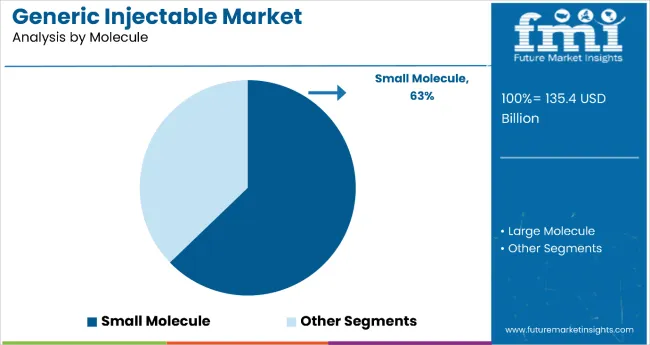
Small molecules dominate the generic injectable market with 62.8% of the market share in the molecule category. This substantial leadership is attributed to small molecule drugs' established manufacturing processes, chemical synthesis capabilities, and ability to penetrate cells effectively to elicit precise biological responses. Small molecules typically offer advantages including high bioavailability, rapid onset of action, and cost-effective production methods that make them accessible across diverse therapeutic applications and healthcare settings.
The segment's dominance is reinforced by small molecules' versatility in treating multiple disease conditions including oncology, infectious diseases, diabetes, and cardiovascular disorders through both systemic distribution and targeted cellular penetration. The increasing prevalence of chronic diseases requiring immediate and effective treatments supports sustained demand for small molecule injectables, while advancements in chemical synthesis and recombinant production techniques continue to enhance their therapeutic efficacy. As healthcare providers prioritize proven, cost-effective treatment options and pharmaceutical companies focus on scalable manufacturing processes, the small molecule segment is expected to maintain its dominant position through continued innovation in drug formulations and delivery technology.
The competitive landscape of the generic injectable market is characterized by the presence of several key players, including both established medical device manufacturers and emerging companies focused on innovative technologies.
Major players such as Erchonia Corporation, Theralase Inc., and Apira Science Inc. are leading the market with advanced laser devices, while smaller companies are increasingly entering the space, offering specialized products.
Competitive strategies primarily revolve around technological innovation, product differentiation, and expanding applications, with a growing emphasis on affordability and accessibility.
| Report Attributes | Details |
|---|---|
| Current Total Market Size (2025) | USD 135.4 billion |
| Projected Market Size (2035) | USD 402.1 billion |
| CAGR (2025 to 2035) | 11.5% |
| Base Year for Estimation | 2024 |
| Historical Period | 2020 to 2024 |
| Projections Period | 2025 to 2035 |
| Analysis Parameter | Revenue in USD Billion |
| By Product | Monoclonal Antibodies, Immunoglobulin, Cytokines, Insulin, Peptide Hormones, Blood Factors, Peptide Antibiotics, Vaccines, Small Molecule Antibiotics, Chemotherapy Agents, Others |
| By Molecule | Small Molecule, Large Molecule |
| By Application | Oncology, Infectious Diseases, Diabetes, Blood Disorders, Hormonal Disorders, Musculoskeletal Disorders, CNS Diseases, Pain Management, Cardiovascular Diseases |
| By Route of Administration | Intravenous (IV), Intramuscular (IM), Subcutaneous (SC), Homecare Settings |
| By Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Drug Stores, Online Pharmacies |
| Regions Covered | North America, Latin America, East Asia, South Asia and Pacific, Western Europe, Eastern Europe, Middle East and Africa |
| Countries Covered | United States, Japan, Germany, India, United Kingdom, France, Italy, Brazil, Canada, South Korea, Australia, Spain, Netherlands, Saudi Arabia, Switzerland |
| Key Players | Samsung Biologics Co Ltd, Aurobindo Pharma Limited, Sun Pharmaceutical Industries Ltd., Novartis AG, Merck & Co., Cipla Ltd, Pfizer Inc, Fresenius Kabi, Sanofi S.A, AstraZeneca Plc, Teva Pharmaceuticals, Mylan N.V., Baxter International, Dr. Reddy’s Laboratories |
| Additional Attributes | Rising demand for biosimilars, increasing prevalence of chronic diseases, growing hospital infrastructure, regulatory support |
| Customization and Pricing | Available upon request |
In terms of product type, the industry is divided into monoclonal antibodies, immunoglobulin, cytokines, insulin, peptide hormones, blood factors, peptide antibiotics, vaccines, small molecule antibiotics, chemotherapy agents, and others .
In terms of molecule, the industry is divided into small molecule, large molecule
In terms of application, the industry is divided into oncology, infectious diseases, diabetes, blood disorders, hormonal disorders, musculoskeletal disorders, CNS diseases, pain management, cardiovascular diseases
In terms of technology, the industry is divided into single wavelength generic injectable devices and multiple wavelength generic injectable devices
In terms of distribution channel, the industry is segregated into intravenous (IV), intramuscular (IM), subcutaneous (SC), homecare settings.
Key countries of North America, Latin America, East Asia, South Asia & Pacific, Western Europe, Eastern Europe, and Middle East and Africa (MEA) have been covered in the report.
The global generic injectable industry is projected to witness CAGR of 11.5% between 2025 and 2035.
The global generic injectable industry stood at USD 120.7 billion in 2024.
The global generic injectable industry is anticipated to reach USD 402.1 billion by 2035 end.
China is expected to show a CAGR of 13.1% in the assessment period.
The key players operating in the global generic injectable industry include Samsung Biologics Co Ltd, Aurobindo Pharma Limited, Sun Pharmaceutical Industries Ltd., Novartis AG, Merck, Cipla Ltd, Pfizer Inc, Fresenius Kabi, Sanofi S.A, AstraZeneca Plc, Teva Pharmaceuticals, Mylan N.A, Baxter International and Dr. Reddy’s Laboratories.
Table 1: Global Market Value (US$ Billion) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 3: Global Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 4: Global Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 5: Global Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 6: Global Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 7: North America Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 8: North America Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 9: North America Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 10: North America Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 11: North America Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 12: North America Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 13: Latin America Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 14: Latin America Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 15: Latin America Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 16: Latin America Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 17: Latin America Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 18: Latin America Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 19: Europe Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 20: Europe Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 21: Europe Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 22: Europe Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 23: Europe Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 24: Europe Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 25: South Asia Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 26: South Asia Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 27: South Asia Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 28: South Asia Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 29: South Asia Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 30: South Asia Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 31: MEA Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 32: MEA Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 33: MEA Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 34: MEA Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 35: MEA Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 36: MEA Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 37: East Asia Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 38: East Asia Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 39: East Asia Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 40: East Asia Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 41: East Asia Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 42: East Asia Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Table 43: Oceania Market Value (US$ Billion) Forecast by Country, 2018 to 2033
Table 44: Oceania Market Value (US$ Billion) Forecast by Product Type, 2018 to 2033
Table 45: Oceania Market Value (US$ Billion) Forecast by Molecule Type, 2018 to 2033
Table 46: Oceania Market Value (US$ Billion) Forecast by Application , 2018 to 2033
Table 47: Oceania Market Value (US$ Billion) Forecast by Route Of Administration, 2018 to 2033
Table 48: Oceania Market Value (US$ Billion) Forecast by Distribution Channel, 2018 to 2033
Figure 1: Global Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 2: Global Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 3: Global Market Value (US$ Billion) by Application , 2023 to 2033
Figure 4: Global Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 5: Global Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 6: Global Market Value (US$ Billion) by Region, 2023 to 2033
Figure 7: Global Market Value (US$ Billion) Analysis by Region, 2018 to 2033
Figure 8: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 9: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 10: Global Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 11: Global Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 12: Global Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 13: Global Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 14: Global Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 15: Global Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 16: Global Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 17: Global Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 18: Global Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 19: Global Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 20: Global Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 21: Global Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 22: Global Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 23: Global Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 24: Global Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 25: Global Market Attractiveness by Product Type, 2023 to 2033
Figure 26: Global Market Attractiveness by Molecule Type, 2023 to 2033
Figure 27: Global Market Attractiveness by Application , 2023 to 2033
Figure 28: Global Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 29: Global Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 30: Global Market Attractiveness by Region, 2023 to 2033
Figure 31: North America Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 32: North America Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 33: North America Market Value (US$ Billion) by Application , 2023 to 2033
Figure 34: North America Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 35: North America Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 36: North America Market Value (US$ Billion) by Country, 2023 to 2033
Figure 37: North America Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 38: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 39: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 40: North America Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 41: North America Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 42: North America Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 43: North America Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 44: North America Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 45: North America Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 46: North America Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 47: North America Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 48: North America Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 49: North America Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 50: North America Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 51: North America Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 52: North America Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 53: North America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 54: North America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 55: North America Market Attractiveness by Product Type, 2023 to 2033
Figure 56: North America Market Attractiveness by Molecule Type, 2023 to 2033
Figure 57: North America Market Attractiveness by Application , 2023 to 2033
Figure 58: North America Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 59: North America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 60: North America Market Attractiveness by Country, 2023 to 2033
Figure 61: Latin America Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 62: Latin America Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 63: Latin America Market Value (US$ Billion) by Application , 2023 to 2033
Figure 64: Latin America Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 65: Latin America Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 66: Latin America Market Value (US$ Billion) by Country, 2023 to 2033
Figure 67: Latin America Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 68: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 69: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 70: Latin America Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 71: Latin America Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 72: Latin America Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 73: Latin America Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 74: Latin America Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 75: Latin America Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 76: Latin America Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 77: Latin America Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 78: Latin America Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 79: Latin America Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 80: Latin America Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 81: Latin America Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 82: Latin America Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 83: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 84: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 85: Latin America Market Attractiveness by Product Type, 2023 to 2033
Figure 86: Latin America Market Attractiveness by Molecule Type, 2023 to 2033
Figure 87: Latin America Market Attractiveness by Application , 2023 to 2033
Figure 88: Latin America Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 89: Latin America Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 90: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 91: Europe Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 92: Europe Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 93: Europe Market Value (US$ Billion) by Application , 2023 to 2033
Figure 94: Europe Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 95: Europe Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 96: Europe Market Value (US$ Billion) by Country, 2023 to 2033
Figure 97: Europe Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 98: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 99: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 100: Europe Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 101: Europe Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 102: Europe Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 103: Europe Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 104: Europe Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 105: Europe Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 106: Europe Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 107: Europe Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 108: Europe Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 109: Europe Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 110: Europe Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 111: Europe Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 112: Europe Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 113: Europe Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 114: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 115: Europe Market Attractiveness by Product Type, 2023 to 2033
Figure 116: Europe Market Attractiveness by Molecule Type, 2023 to 2033
Figure 117: Europe Market Attractiveness by Application , 2023 to 2033
Figure 118: Europe Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 119: Europe Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 120: Europe Market Attractiveness by Country, 2023 to 2033
Figure 121: South Asia Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 122: South Asia Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 123: South Asia Market Value (US$ Billion) by Application , 2023 to 2033
Figure 124: South Asia Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 125: South Asia Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 126: South Asia Market Value (US$ Billion) by Country, 2023 to 2033
Figure 127: South Asia Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 128: South Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 129: South Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 130: South Asia Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 131: South Asia Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 132: South Asia Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 133: South Asia Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 134: South Asia Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 135: South Asia Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 136: South Asia Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 137: South Asia Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 138: South Asia Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 139: South Asia Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 140: South Asia Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 141: South Asia Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 142: South Asia Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 143: South Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 144: South Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 145: South Asia Market Attractiveness by Product Type, 2023 to 2033
Figure 146: South Asia Market Attractiveness by Molecule Type, 2023 to 2033
Figure 147: South Asia Market Attractiveness by Application , 2023 to 2033
Figure 148: South Asia Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 149: South Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 150: South Asia Market Attractiveness by Country, 2023 to 2033
Figure 151: MEA Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 152: MEA Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 153: MEA Market Value (US$ Billion) by Application , 2023 to 2033
Figure 154: MEA Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 155: MEA Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 156: MEA Market Value (US$ Billion) by Country, 2023 to 2033
Figure 157: MEA Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 158: MEA Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 159: MEA Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 160: MEA Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 161: MEA Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 162: MEA Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 163: MEA Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 164: MEA Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 165: MEA Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 166: MEA Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 167: MEA Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 168: MEA Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 169: MEA Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 170: MEA Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 171: MEA Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 172: MEA Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 173: MEA Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 174: MEA Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 175: MEA Market Attractiveness by Product Type, 2023 to 2033
Figure 176: MEA Market Attractiveness by Molecule Type, 2023 to 2033
Figure 177: MEA Market Attractiveness by Application , 2023 to 2033
Figure 178: MEA Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 179: MEA Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 180: MEA Market Attractiveness by Country, 2023 to 2033
Figure 181: East Asia Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 182: East Asia Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 183: East Asia Market Value (US$ Billion) by Application , 2023 to 2033
Figure 184: East Asia Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 185: East Asia Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 186: East Asia Market Value (US$ Billion) by Country, 2023 to 2033
Figure 187: East Asia Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 188: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 189: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 190: East Asia Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 191: East Asia Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 192: East Asia Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 193: East Asia Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 194: East Asia Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 195: East Asia Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 196: East Asia Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 197: East Asia Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 198: East Asia Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 199: East Asia Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 200: East Asia Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 201: East Asia Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 202: East Asia Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 203: East Asia Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 204: East Asia Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 205: East Asia Market Attractiveness by Product Type, 2023 to 2033
Figure 206: East Asia Market Attractiveness by Molecule Type, 2023 to 2033
Figure 207: East Asia Market Attractiveness by Application , 2023 to 2033
Figure 208: East Asia Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 209: East Asia Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 210: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 211: Oceania Market Value (US$ Billion) by Product Type, 2023 to 2033
Figure 212: Oceania Market Value (US$ Billion) by Molecule Type, 2023 to 2033
Figure 213: Oceania Market Value (US$ Billion) by Application , 2023 to 2033
Figure 214: Oceania Market Value (US$ Billion) by Route Of Administration, 2023 to 2033
Figure 215: Oceania Market Value (US$ Billion) by Distribution Channel, 2023 to 2033
Figure 216: Oceania Market Value (US$ Billion) by Country, 2023 to 2033
Figure 217: Oceania Market Value (US$ Billion) Analysis by Country, 2018 to 2033
Figure 218: Oceania Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 219: Oceania Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 220: Oceania Market Value (US$ Billion) Analysis by Product Type, 2018 to 2033
Figure 221: Oceania Market Value Share (%) and BPS Analysis by Product Type, 2023 to 2033
Figure 222: Oceania Market Y-o-Y Growth (%) Projections by Product Type, 2023 to 2033
Figure 223: Oceania Market Value (US$ Billion) Analysis by Molecule Type, 2018 to 2033
Figure 224: Oceania Market Value Share (%) and BPS Analysis by Molecule Type, 2023 to 2033
Figure 225: Oceania Market Y-o-Y Growth (%) Projections by Molecule Type, 2023 to 2033
Figure 226: Oceania Market Value (US$ Billion) Analysis by Application , 2018 to 2033
Figure 227: Oceania Market Value Share (%) and BPS Analysis by Application , 2023 to 2033
Figure 228: Oceania Market Y-o-Y Growth (%) Projections by Application , 2023 to 2033
Figure 229: Oceania Market Value (US$ Billion) Analysis by Route Of Administration, 2018 to 2033
Figure 230: Oceania Market Value Share (%) and BPS Analysis by Route Of Administration, 2023 to 2033
Figure 231: Oceania Market Y-o-Y Growth (%) Projections by Route Of Administration, 2023 to 2033
Figure 232: Oceania Market Value (US$ Billion) Analysis by Distribution Channel, 2018 to 2033
Figure 233: Oceania Market Value Share (%) and BPS Analysis by Distribution Channel, 2023 to 2033
Figure 234: Oceania Market Y-o-Y Growth (%) Projections by Distribution Channel, 2023 to 2033
Figure 235: Oceania Market Attractiveness by Product Type, 2023 to 2033
Figure 236: Oceania Market Attractiveness by Molecule Type, 2023 to 2033
Figure 237: Oceania Market Attractiveness by Application , 2023 to 2033
Figure 238: Oceania Market Attractiveness by Route Of Administration, 2023 to 2033
Figure 239: Oceania Market Attractiveness by Distribution Channel, 2023 to 2033
Figure 240: Oceania Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
India Generic Injectable Market Analysis – Growth, Applications & Outlook 2025-2035
China Generic Injectable Market Trends – Growth, Demand & Forecast 2025-2035
Germany Generic Injectable Market Outlook – Share, Growth & Forecast 2025-2035
United States Generic Injectable Market Report – Demand, Trends & Industry Forecast 2025-2035
United Kingdom Generic Injectable Market Trends – Size, Share & Growth 2025-2035
Generic Oncology Drugs Market Growth – Industry Forecast 2025 to 2035
The Super Generics Market Is Segmented by Drug Type, Therapeutic Area, Route of Administration and Distribution Channel from 2025 To 2035
Super Generics Industry Analysis in Europe Report - Trends & Innovations 2025 to 2035
Complex Generics Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Branded Generics Market Analysis - Size, Share, and Forecast Outlook from 2025 to 2035
Specialty Generics Market
Europe and MENA Generic Oncology Drug Market Size and Share Forecast Outlook 2025 to 2035
Inhalation And Nasal Spray Generic Drugs Market Size and Share Forecast Outlook 2025 to 2035
Injectable Anti-Wrinkle Treatment Market Size and Share Forecast Outlook 2025 to 2035
Injectable Thyroid Drug Market Size and Share Forecast Outlook 2025 to 2035
Injectable Nanomedicines Market Size and Share Forecast Outlook 2025 to 2035
Injectable Liquid Filling Machines Market Size and Share Forecast Outlook 2025 to 2035
Injectable Drug Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Injectable Potassium Phosphate Market – Growth & Forecast 2024 to 2034
Injectable Drug Delivery Market Analysis – Growth & Trends 2024-2034

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA