The investigational new drug (IND) CDMO market is valued at USD 5.7 billion in 2025 and is projected to reach USD 13.0 billion by 2035, with a CAGR of 8.7%. From 2021 to 2025, the market grows steadily from USD 3.7 billion to USD 5.7 billion, moving through intermediate values of USD 4.1 billion, 4.4 billion, and 4.8 billion. This period is driven by the increasing demand for contract manufacturing organizations (CMOs) specializing in the development of investigational new drugs for clinical trials. With the rising number of drug candidates entering early-stage clinical trials, pharmaceutical companies rely more on external manufacturing capabilities to meet the complex demands of drug development and regulatory compliance.
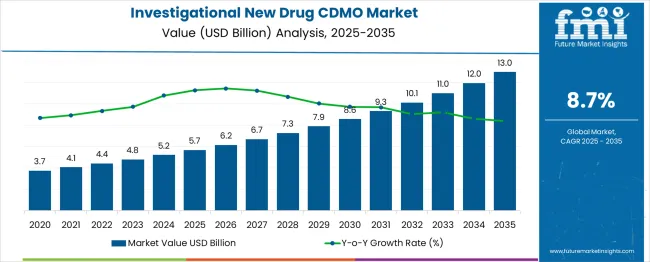
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 5.7 billion |
| Forecast Value in (2035F) | USD 13 billion |
| Forecast CAGR (2025 to 2035) | 8.7 % |
The investigational new drug (IND) CDMO market is primarily influenced by several parent markets that provide critical services throughout the drug development process. The pharmaceutical contract manufacturing market contributes around 20-25%, as CDMOs play a crucial role in the manufacturing of active pharmaceutical ingredients (APIs) and final drug formulations. These services are essential for developing investigational drugs, ensuring they are ready for clinical trials. The pharmaceutical development services market contributes approximately 18-22%, encompassing the early-stage services necessary for IND development, such as formulation development, clinical trial material production, and stability testing. This segment is vital for ensuring that investigational drugs are prepared and optimized for clinical trials.
The biopharmaceutical contract manufacturing market represents about 15-18%, driven by the increasing demand for the production of biologics, gene therapies, and monoclonal antibodies, which require specialized manufacturing capabilities offered by CDMOs. The clinical trial services market contributes around 12-15%, as CDMOs often collaborate with clinical trial organizations to supply investigational products and materials for use in clinical studies. The regulatory and compliance services market, contributing 8-10%, provides essential support for IND submission and approval processes. These services ensure that investigational drugs meet all regulatory standards, guiding CDMOs through the regulatory complexities of clinical trial approvals, documentation, and submission.
Market expansion is being supported by the increasing pharmaceutical industry focus on core competencies and the corresponding demand for specialized outsourcing services. Modern pharmaceutical companies are increasingly focused on research and development while outsourcing manufacturing and development activities to reduce costs, access specialized expertise, and accelerate product development timelines. CDMO providers' proven capabilities in regulatory compliance, quality assurance, and specialized manufacturing make them preferred partners for complex drug development programs.
The growing focus on regulatory compliance and quality standards is driving demand for CDMO services that can navigate complex regulatory landscapes across different markets. Pharmaceutical companies' preference for integrated service offerings that combine development, manufacturing, and regulatory support is creating opportunities for comprehensive CDMO solutions. The rising influence of personalized medicine and complex biologics is also contributing to increased demand for specialized manufacturing capabilities across different therapeutic areas and drug modalities.
The market is segmented by service outlook, end-use outlook, and region. By service outlook, the market is divided into contract development, small molecule formulation development, analytical & quality services, process optimization, large molecule manufacturing, small molecule manufacturing, and large molecule development. Based on end-use outlook, the market is categorized into pharmaceutical companies, biotech companies, and others. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and the Middle East & Africa.
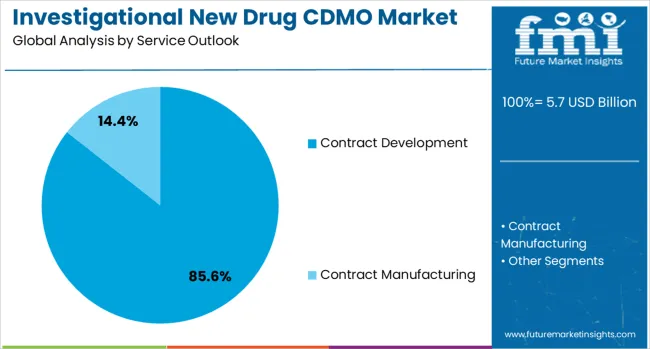
The contract development service is projected to account for 85.6 % of the investigational new drug CDMO market in 2025, reaffirming its position as the category's dominant service offering. Pharmaceutical companies increasingly understand the value of partnering with specialized providers for drug development activities, including formulation development, analytical testing, process optimization, and regulatory support. CDMO providers' comprehensive development capabilities directly address industry needs by providing expertise, reducing development timelines, and ensuring regulatory compliance.
This service forms the foundation of most CDMO partnerships, as it represents the most critical and complex aspect of drug development outsourcing. Regulatory expertise and proven track records continue to strengthen trust in CDMO development services. With pharmaceutical companies focusing on core research activities while outsourcing development functions, contract development aligns with both cost optimization and efficiency improvement goals. Its broad appeal across pharmaceutical and biotech sectors ensures dominance, making it the central growth driver of investigational new drug CDMO demand.
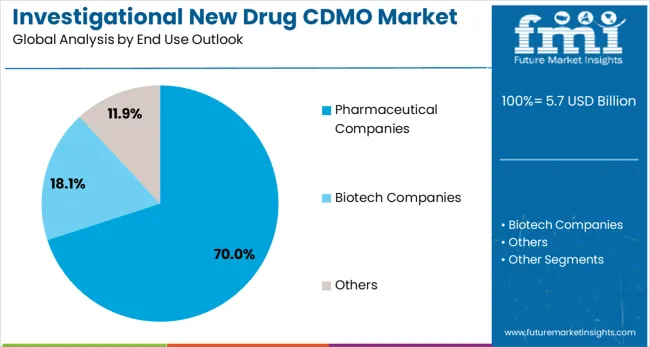
Pharmaceutical companies are projected to represent 70 % of investigational new drug CDMO demand in 2025, underscoring their role as the primary customer base for specialized development and manufacturing services. Large pharmaceutical companies gravitate toward CDMO partnerships for their ability to provide specialized expertise, reduce operational costs, and accelerate development timelines while maintaining focus on core research and commercial activities. Positioned as strategic partners, CDMOs offer both development support and manufacturing capabilities that enable pharmaceutical companies to bring new drugs to market more efficiently.
The segment is supported by the rising trend of pharmaceutical companies focusing on their core competencies while outsourcing non-core activities to specialized service providers. Pharmaceutical companies are increasingly seeking integrated service offerings that combine development, manufacturing, and regulatory support, enhancing operational efficiency and justifying partnership investments. As pharmaceutical companies prioritize speed-to-market and cost optimization, partnerships with investigational new drug CDMOs will continue to dominate demand, reinforcing their position as the primary end user segment.
The investigational new drug CDMO market is advancing rapidly due to increasing pharmaceutical industry outsourcing trends and growing demand for specialized development and manufacturing services. The market faces challenges including regulatory complexity, quality assurance requirements, and competition among service providers. Innovation in technology integration and expansion of service capabilities continue to influence market development and competitive positioning patterns.
The growing demand for comprehensive CDMO partnerships is enabling providers to offer integrated services spanning development, manufacturing, and regulatory support. Integrated service models provide pharmaceutical companies with streamlined project management, reduced complexity, and improved communication throughout the drug development process. Strategic partnerships and long-term relationships are driving service expansion and enabling CDMOs to provide more comprehensive solutions to their pharmaceutical clients.
AI, process analytical technology (PAT), and continuous manufacturing systems are being increasingly implemented to streamline development timelines and ensure the consistency of product quality. These technologies not only enable faster and more accurate drug development but also facilitate compliance with stringent regulatory requirements. The use of digital platforms is enhancing communication and project management, allowing pharmaceutical companies to track development progress and manufacturing status in real-time. This digital transformation is significantly improving operational transparency and fostering stronger collaboration between CDMOs and their clients, optimizing the overall drug development process.
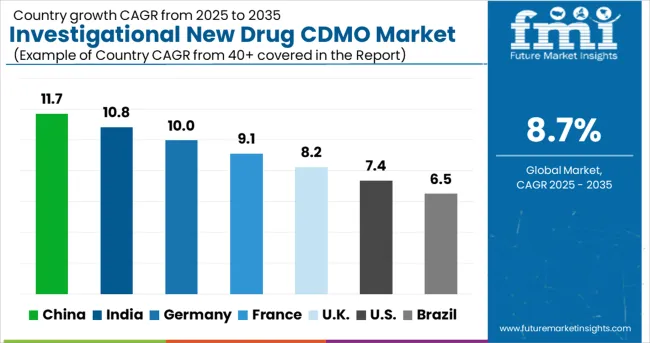
| Countries | CAGR (2025-2035) |
|---|---|
| China | 11.7% |
| India | 10.8% |
| Germany | 10 % |
| France | 9.1% |
| UK | 8.2% |
| USA | 7.4% |
| Brazil | 6.5% |
The global investigational new drug (IND) CDMO market is expected to grow at a CAGR of 8.7% from 2025 to 2035. China leads the market with an 11.7% CAGR, driven by expanding pharmaceutical manufacturing capabilities and increasing involvement in global drug development programs. India follows closely with a 10.8% CAGR, benefiting from its cost-effective services and compliance with international quality standards. Germany experiences steady growth at a 10% CAGR, showing advanced manufacturing technologies and regulatory expertise. France shows growth at 9.1% CAGR, focusing on specialized development services and pharmaceutical industry partnerships. The UK shows an 8.2% CAGR, prioritizing innovation and regulatory compliance in drug development services.
The report covers an in-depth analysis of 40+ countries, with top-performing markets showing below.
Demand for investigational new drug CDMO services in China is projected to exhibit strong growth with a CAGR of 11.7% through 2035, driven by the rapid expansion of pharmaceutical manufacturing capabilities and increasing participation in global drug development programs. The country's growing pharmaceutical industry and improving regulatory environment are creating significant demand for specialized CDMO services. Major international and domestic CDMO providers are establishing comprehensive service capabilities to serve the growing population of pharmaceutical companies seeking cost-effective and high-quality development and manufacturing solutions.
The investigational new drug CDMO services market in India is expanding at a CAGR of 10.8%, supported by established pharmaceutical manufacturing expertise, competitive cost structures, and increasing compliance with international quality standards. The country's strong pharmaceutical industry foundation and skilled workforce are driving demand for comprehensive CDMO services. International pharmaceutical companies and domestic manufacturers are expanding partnerships with Indian CDMO providers to access cost-effective development and manufacturing capabilities.
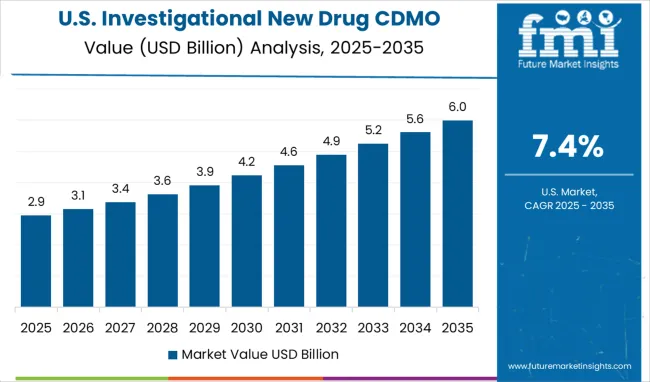
Demand for investigational new drug CDMO services in the USA is projected to grow at a CAGR of 7.4%, supported by the pharmaceutical industry's focus on outsourcing non-core activities and accessing specialized expertise. American pharmaceutical companies are increasingly focused on core research and development while partnering with CDMO providers for manufacturing and specialized development services. The market is characterized by strong demand for integrated service offerings that combine development expertise with regulatory compliance and manufacturing capabilities.
Revenue from investigational new drug CDMO services in Germany is projected to grow at a CAGR of 10 % through 2035. The strong regulatory environment, coupled with its leadership in clinical research, creates a solid foundation for the growth of the IND CDMO market. Germany's established pharmaceutical and biotech sectors continue to thrive, with a growing focus on biologics and personalized medicine. The integration of cutting-edge technologies in drug development, such as gene therapy and biologic manufacturing, is pushing the demand for specialized CDMO services. With a focus on high-quality standards and regulatory compliance, Germany remains a trusted location for global drug developers.
The investigational new drug CDMO services market in the UK is projected to grow at a CAGR of 8.2% through 2035. The country’s pharmaceutical sector is bolstered by a strong regulatory framework, which encourages international collaborations and clinical research. The UK’s National Health Service (NHS) plays a key role in integrating drug development services, making it an attractive market for IND CDMO providers. The expertise in biologics and biosimilars is further driving market demand. The growth of the biotech industry, along with increasing funding for drug development initiatives, is providing more opportunities for IND CDMO companies to expand their services.
Revenue from investigational new drug CDMO services in France is projected to grow at a CAGR of 9.1% through 2035. The country continues to lead in drug innovation, supported by its well-established pharmaceutical infrastructure, which includes world-class regulatory support and advanced manufacturing capabilities. The USA is also home to many of the world’s largest pharmaceutical companies, biotech firms, and research institutions, making it a hub for drug development and clinical trials. The increasing demand for personalized medicine and biologics is a key factor driving growth in the IND CDMO market. The USA is at the forefront of advancements in gene therapy, which is creating new opportunities for CDMO providers.
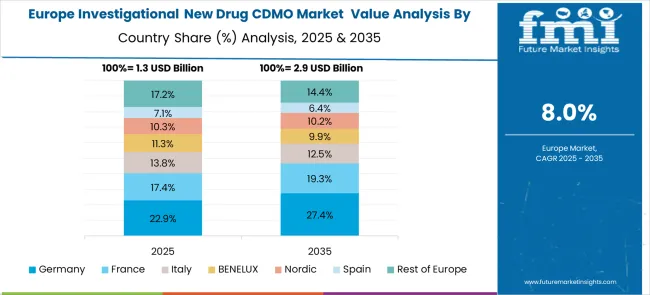
The investigational new drug CDMO market in Europe demonstrates mature development across major economies with Germany showing strong presence through its advanced pharmaceutical manufacturing infrastructure and regulatory expertise, supported by established CDMO providers leveraging scientific excellence to develop comprehensive drug development and manufacturing services that meet stringent European regulatory standards and quality requirements.
France represents a significant market driven by its pharmaceutical industry heritage and sophisticated understanding of drug development processes, with companies focusing on specialized manufacturing capabilities and regulatory compliance services that combine French pharmaceutical expertise with advanced CDMO service delivery for enhanced development efficiency and market access support.
The UK exhibits considerable growth through its strong pharmaceutical sector and expertise in drug development, with established CDMO providers leading innovation in specialized manufacturing services and comprehensive regulatory support solutions. Italy and Spain show expanding interest in CDMO partnerships, particularly in specialized development services targeting complex drug formulations and manufacturing optimization. BENELUX countries contribute through their focus on high-quality manufacturing and regulatory compliance services, while Eastern Europe and Nordic regions display growing potential driven by increasing pharmaceutical outsourcing activities and expanding access to specialized CDMO services across diverse therapeutic areas.
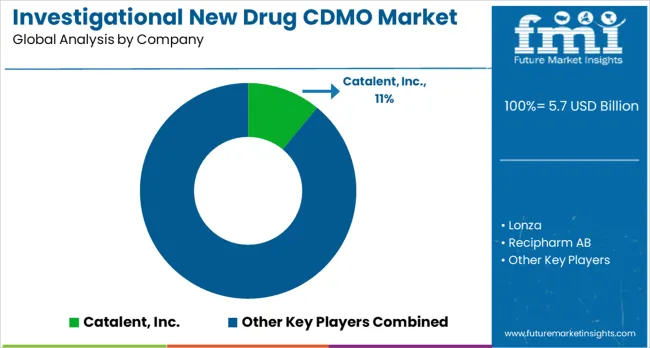
The investigational new drug (IND) contract development and manufacturing organization (CDMO) market is marked by robust competition as companies invest in cutting-edge manufacturing technologies, integrated services, regulatory expertise, and global expansion strategies. These investments are aimed at delivering efficient, compliant, and comprehensive drug development and manufacturing solutions. The key drivers in this space are service differentiation, technological advancements, and geographic expansion, all of which help strengthen service offerings and improve market positioning.
Catalent Inc., based in the USA, is a leading player in the market with an 11% global value share. The company offers a broad range of CDMO services, with a focus on integrated development and manufacturing capabilities across various therapeutic areas. Lonza, headquartered in Switzerland, specializes in biologics and complex pharmaceutical products, providing services that cater to the growing demand for biologics in drug development. Recipharm, based in Sweden, provides a comprehensive range of pharmaceutical development and manufacturing services, with a strong focus on regulatory compliance and quality assurance, positioning itself as a key player in the industry. Siegfried Holding AG, also based in Switzerland, focuses on specialized manufacturing and development services for complex pharmaceutical products.
Patheon Inc., a part of Thermo Fisher Scientific, offers a wide array of CDMO services across multiple pharmaceutical segments and geographic regions. This global reach positions Patheon as one of the most prominent service providers in the market. Covance and IQVIA Holdings Inc., both operating globally, combine clinical research with development and manufacturing services, enabling them to offer comprehensive, integrated solutions. Cambrex Corporation, based in the USA, specializes in active pharmaceutical ingredient (API) development and production, providing targeted solutions for API manufacturers.
Other notable players, including Charles River Laboratories, Syneos Health, and various specialized providers, focus on specific therapeutic areas and regulatory expertise. Their strategic differentiation lies in offering specialized services designed to cater to diverse drug development needs and ensuring compliance in a highly regulated industry. These companies are integral in shaping the future of the CDMO market through innovation and comprehensive service offerings.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 5.7 billion |
| Service Outlook | Contract Development, Small Molecule Formulation Development, Analytical & Quality Services, Process Optimization, Large Molecule Manufacturing, Small Molecule Manufacturing, Large Molecule Development |
| End Use Outlook | Pharmaceutical Companies, Biotech Companies, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, India, Brazil, Australia and 40+ countries |
| Key Companies Profiled | Catalent, Lonza, Recipharm, Siegfried Holding AG, Patheon Inc., Covance, IQVIA Holdings Inc., Cambrex Corporation, Charles River Laboratories International Inc., Syneos Health, Syngene International, Jubilant Pharmova, Piramal Pharma Solutions, Dr. Reddy's CPS, and Dishman Carbogen Amcis |
| Additional Attributes | Revenue analysis by service type and complexity level, regional demand trends, competitive landscape, client preferences for integrated versus specialized services, regulatory compliance requirements, innovations in manufacturing technologies, process optimization, and quality assurance practices |
The global investigational new drug cdmo market is estimated to be valued at USD 5.7 billion in 2025.
The market size for the investigational new drug cdmo market is projected to reach USD 13.0 billion by 2035.
The investigational new drug cdmo market is expected to grow at a 8.7% CAGR between 2025 and 2035.
The key product types in investigational new drug cdmo market are contract development, small molecule, formulation development, analytical & quality services, process optimization, large molecule, contract manufacturing, small molecule and large molecule.
In terms of end use outlook, pharmaceutical companies segment to command 70.0% share in the investigational new drug cdmo market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Newborn Identification Tag Market Size and Share Forecast Outlook 2025 to 2035
Newborn Jaundice Treatment Market Size and Share Forecast Outlook 2025 to 2035
Newborn Name Tag Market Size and Share Forecast Outlook 2025 to 2035
New Energy Vehicle Electric Drive Systems Market Size and Share Forecast Outlook 2025 to 2035
New Zealand Sustainable Tourism Market Size and Share Forecast Outlook 2025 to 2035
New Born Eye Imaging Systems Market Size and Share Forecast Outlook 2025 to 2035
New-born Screening Equipment Market - Growth & Demand 2025 to 2035
New Zealand Sports Tourism Market Analysis - Size, Share, and Forecast 2025 to 2035
Renewable Heating Fuels Market Size and Share Forecast Outlook 2025 to 2035
Renewable Isocyanate Market Forecast and Outlook 2025 to 2035
Renewables Energy Consulting Service Market Size and Share Forecast Outlook 2025 to 2035
Renewable Naphtha Market Size and Share Forecast Outlook 2025 to 2035
Renewable Biopolymer Cosmetics Market Size and Share Forecast Outlook 2025 to 2035
Renewable Based Shunt Reactor Market Size and Share Forecast Outlook 2025 to 2035
Renewable Energy Certificate Market Size and Share Forecast Outlook 2025 to 2035
Renewable Polyethylene Market Size and Share Forecast Outlook 2025 to 2035
Renewable Energy Contactor Market Size and Share Forecast Outlook 2025 to 2035
Renewable Methanol Market Growth - Trends & Forecast 2025 to 2035
Renewable Solvents Market
Cellular Renewal Actives Market Analysis - Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA