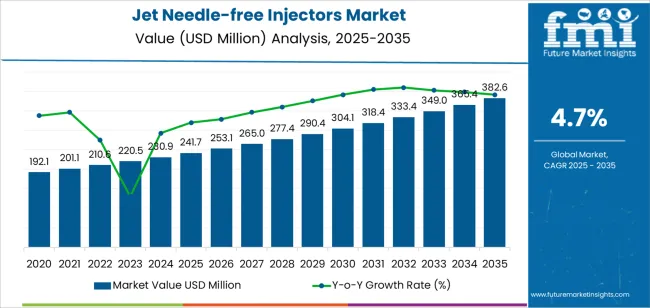
The jet needle-free injectors market is expected to grow from USD 241.7 million in 2025 to USD 382.6 million by 2035, with market momentum shaped by shifting priorities in patient comfort, infection control, and home-based treatment models. A primary trend shaping adoption is the transition from needle-based injections to pain-reduced, pressure-driven liquid delivery systems, particularly in diabetes and chronic care routines where daily or frequent dosing increases patient fatigue and needle anxiety. The growing emphasis on needle-stick injury prevention in hospitals and clinical settings is also accelerating institutional acceptance, as healthcare worker safety protocols increasingly discourage sharps usage unless necessary.
Another key trend is the emergence of self-administration and home-care treatment platforms, where compact, user-friendly needle-free injectors support decentralization of treatment from hospital environments to personal care routines. Pharmaceutical companies are also beginning to co-develop drug–device integrated delivery models, particularly for biologics, peptides, and vaccines requiring controlled intradermal or subcutaneous dispersion. At the same time, improvements in nozzle geometry, pressure modulation, and dose precision calibration are increasing clinical confidence in the consistency of jet injection performance. Beyond clinical healthcare, rising demand in mass vaccination and public health immunization programs is positioning needle-free systems as viable tools for high-volume, rapid-deployment injection campaigns.
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | New device sales (liquid, powder, depot systems) | 44% | Healthcare-led, replacement-driven purchases |
| Replacement cartridges & consumables | 26% | Ampoules, pressure cartridges, sterile tips | |
| Pharmaceutical partnerships & licensing | 18% | Drug-device combinations, co-development | |
| Service & maintenance contracts | 12% | Device servicing, patient training support | |
| Future (3-5 yrs) | Connected injection systems | 38-42% | Digital tracking, dose monitoring, compliance data |
| Consumables & cartridges | 24-28% | Recurring revenue, proprietary formats | |
| Pharmaceutical collaborations | 18-22% | Biologic delivery, specialized formulations | |
| Patient support programs | 10-14% | Training services, adherence monitoring | |
| Data analytics services | 5-8% | Usage patterns, compliance tracking | |
| Rental & subscription models | 3-6% | Device-as-a-service for chronic conditions |
The latter half (2030-2035) will witness continued growth from USD 304.1 million to USD 382.6 million, representing an addition of USD 78.5 million or 61% of the decade's expansion. This period will be defined by mass market penetration of powder injection technologies, integration with comprehensive disease management platforms, and seamless compatibility with existing healthcare infrastructure. The market trajectory signals fundamental shifts in how healthcare facilities approach drug delivery and patient safety, with participants positioned to benefit from growing demand across multiple injection types and application segments.
Regional dynamics reveal distinct adoption patterns, with developed markets leading technology advancement while emerging economies show accelerating growth through healthcare modernization programs. North American markets maintain steady expansion supported by diabetes management requirements and patient safety initiatives, while Asian markets demonstrate rapid growth driven by vaccination program expansion and healthcare infrastructure development. European markets show moderate growth influenced by patient-centric care standards and pharmaceutical innovation trends.
Healthcare industry evolution drives fundamental changes in injection delivery specifications, with healthcare providers and patients increasingly prioritizing products that combine delivery precision, safety features, and ease of use. The decade ahead will witness transformation from traditional needle-based systems toward sophisticated needle-free technologies with advanced pressure mechanisms, dose accuracy controls, and ergonomic designs that meet diverse therapeutic and preventive care requirements.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 241.7 million |
| Market Forecast (2035) | USD 382.6 million |
| Growth Rate | 4.7% CAGR |
| Leading Injection Technology | Liquid Injections |
| Primary Application | Vaccine Delivery Segment |
The market demonstrates strong fundamentals with liquid injection systems capturing a dominant share through advanced delivery precision capabilities and pharmaceutical administration optimization. Vaccine delivery applications drive primary demand, supported by increasing immunization programs and mass vaccination requirements. Geographic expansion remains concentrated in developed markets with established healthcare infrastructure, while emerging economies show accelerating adoption rates driven by vaccination initiatives and rising patient safety standards.
Healthcare trends favor jet needle-free injectors that integrate delivery accuracy with patient comfort features, creating opportunities for manufacturers offering comprehensive product portfolios. The decade ahead presents expansion potential across vaccination programs, insulin delivery segments, and self-administration applications, with market participants positioned to capitalize on growing demand for pain-free injection solutions that meet evolving healthcare standards and patient preferences.
Primary Classification: The market segments by injection type into powder injections, liquid injections, and depot or projectile injections, representing the evolution from basic needle-free delivery systems to sophisticated pharmaceutical administration solutions for comprehensive healthcare optimization.
Secondary Classification: Application segmentation divides the market into vaccine delivery, insulin delivery, and other therapeutic sectors, reflecting distinct requirements for dosing precision, administration convenience, and patient compliance standards.
Regional Classification: Geographic distribution covers North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, and Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by healthcare infrastructure expansion programs.
The classification structure reveals technology progression from standard needle-free mechanisms toward sophisticated delivery systems with enhanced dose accuracy and patient safety capabilities, while application diversity spans from vaccination programs to chronic disease management requiring precise pharmaceutical delivery solutions.
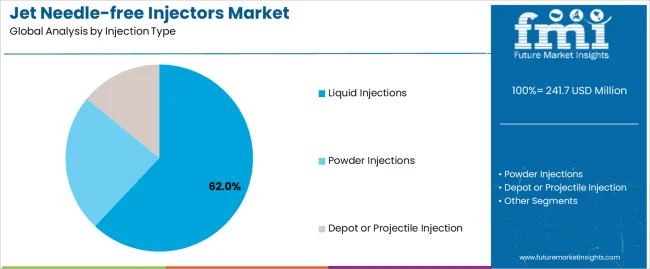
Market Position: Liquid injection systems command the leading position in the market with 62.0% market share through advanced delivery precision features, including superior dose accuracy, pharmaceutical compatibility, and healthcare administration optimization that enable medical facilities to achieve consistent therapeutic outcomes across diverse clinical and self-administration environments.
Value Drivers: The segment benefits from healthcare provider preference for versatile injection systems that provide reliable drug delivery, rapid administration capability, and patient comfort optimization without requiring complex preparation procedures. Advanced design features enable precise volume control, adjustable pressure settings, and integration with various pharmaceutical formulations, where delivery accuracy and patient acceptance represent critical treatment requirements.
Competitive Advantages: Liquid injection systems differentiate through proven delivery reliability, consistent therapeutic outcomes, and compatibility with standard pharmaceutical preparations that enhance treatment effectiveness while maintaining optimal patient safety suitable for diverse clinical applications.
Key market characteristics:
Powder injection systems maintain a 24% market position in the jet needle-free injectors market due to their vaccine delivery advantages and storage stability properties. These systems appeal to immunization programs requiring ambient temperature storage with effective delivery for mass vaccination applications. Market growth is driven by global vaccination expansion, focusing stable formulation solutions and cold chain elimination through optimized powder delivery designs.
Depot or projectile injection systems capture 14% market share through specialized delivery requirements in long-acting medications, controlled release applications, and specialized therapeutic scenarios. These applications demand precise implantation capability capable of delivering solid drug formulations while providing sustained release characteristics and extended therapeutic duration.
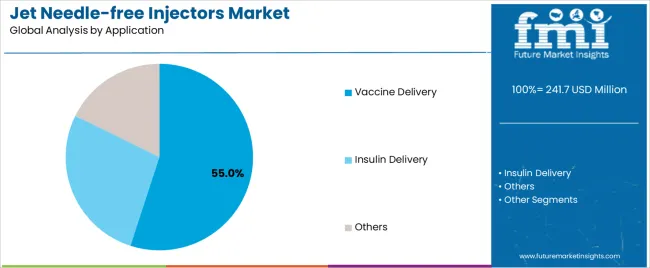
Market Context: Vaccine delivery applications demonstrate the 55.0% market share with 5.3% CAGR due to widespread adoption of mass immunization programs and increasing focus on vaccination coverage expansion, pandemic preparedness, and healthcare accessibility applications that maximize immunization efficiency while maintaining safety standards.
Appeal Factors: Healthcare administrators prioritize system efficiency, rapid deployment capability, and integration with immunization programs that enable coordinated vaccination operations across multiple population segments. The segment benefits from substantial government investment and public health initiatives that emphasize the acquisition of needle-free systems for vaccination campaigns and routine immunization applications.
Growth Drivers: Global immunization programs incorporate needle-free injectors as preferred equipment for mass vaccination, while pandemic preparedness increases demand for rapid deployment capabilities that comply with safety standards and minimize cross-contamination complexity.
Market Challenges: Varying vaccine formulations and cold chain requirements may limit system standardization across different immunization programs or regional scenarios.
Application dynamics include:
Insulin delivery applications capture market share through essential therapeutic requirements in diabetes management, chronic care programs, and self-administration scenarios. These applications demand reliable injection systems capable of operating with daily dosing requirements while providing consistent delivery performance and patient convenience capabilities.
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Rising diabetes prevalence & insulin therapy expansion | ★★★★★ | Growing diabetic populations require convenient self-administration solutions with improved patient compliance and reduced injection anxiety across treatment regimens. |
| Driver | Needlestick injury prevention & healthcare worker safety | ★★★★★ | Occupational safety concerns transform needle-free systems from optional to essential; devices eliminating sharps injuries gain institutional adoption. |
| Driver | Mass vaccination programs & immunization initiatives | ★★★★☆ | Global immunization campaigns need efficient, safe delivery systems; demand for rapid administration and cross-contamination prevention expanding addressable market. |
| Restraint | High device costs & reimbursement limitations | ★★★★☆ | Premium pricing versus traditional syringes creates adoption barriers; limited insurance coverage slows consumer acceptance in price-sensitive markets. |
| Restraint | Pharmaceutical formulation compatibility & stability concerns | ★★★☆☆ | Not all medications suitable for needle-free delivery; formulation requirements and viscosity limitations restrict application breadth. |
| Trend | Biologic drug delivery & high-viscosity formulations | ★★★★★ | Advanced therapeutics require novel delivery methods; needle-free systems enabling biologic administration become critical value propositions. |
| Trend | Digital health integration & connected devices | ★★★★☆ | Smart injectors with dose tracking and adherence monitoring; data connectivity and patient engagement features drive competition toward integrated solutions. |
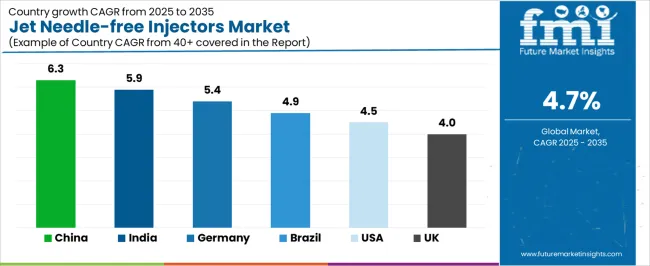
The jet needle-free injectors market demonstrates varied regional dynamics with Growth Leaders including China (6.3% growth rate) and India (5.9% growth rate) driving expansion through healthcare infrastructure initiatives and vaccination program development. Steady Performers encompass Germany (5.4% growth rate), Brazil (4.9% growth rate), and developed regions, benefiting from established healthcare systems and patient safety adoption. Mature Markets feature United States (4.5% growth rate) and United Kingdom (4.0% growth rate), where diabetes management programs and healthcare innovation support consistent growth patterns.
Regional synthesis reveals East Asian markets leading adoption through healthcare expansion and pharmaceutical development, while North American countries maintain steady expansion supported by chronic disease management and regulatory standardization requirements. European markets show moderate growth driven by patient safety applications and healthcare quality integration trends.
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| China | 6.3% | Focus on vaccination programs | Regulatory approval complexity |
| India | 5.9% | Offer affordable solutions | Distribution infrastructure gaps |
| Germany | 5.4% | Lead with precision systems | Reimbursement negotiations |
| Brazil | 4.9% | Value-oriented devices | Economic fluctuations |
| USA | 4.5% | Provide clinical evidence | Insurance coverage variability |
| UK | 4.0% | Push patient safety benefits | NHS procurement cycles |
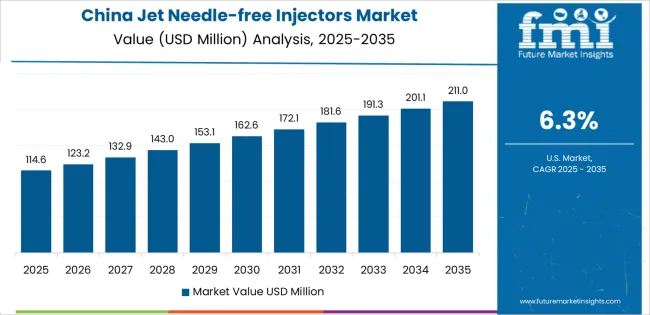
China establishes fastest market growth through aggressive healthcare modernization programs and comprehensive vaccination infrastructure development, integrating jet needle-free injectors as standard components in immunization facilities and hospital administration systems. The country's 6.3% growth rate reflects government initiatives promoting healthcare quality advancement and patient safety capabilities that mandate the use of advanced injection systems in vaccination and pharmaceutical delivery facilities. Growth concentrates in major healthcare centers, including Beijing, Shanghai, and Guangzhou, where vaccination programs showcase integrated needle-free systems that appeal to healthcare administrators seeking efficient immunization capabilities and safety enhancement applications.
Chinese manufacturers are developing cost-effective injection solutions that combine domestic production advantages with improving safety features, including contamination prevention and dose accuracy capabilities. Distribution channels through medical equipment suppliers and pharmaceutical distributors expand market access, while government support for vaccination programs supports adoption across diverse healthcare and public health segments.
Strategic Market Indicators:
In Mumbai, Delhi, and Bangalore, healthcare facilities and vaccination centers are implementing jet needle-free injectors as standard equipment for immunization and pharmaceutical delivery applications, driven by increasing government healthcare investment and infrastructure modernization programs that emphasize the importance of patient safety capabilities. The market holds a 5.9% growth rate, supported by government immunization initiatives and healthcare infrastructure development programs that promote advanced injection systems for vaccination and therapeutic facilities. Indian healthcare providers are adopting injection systems that provide reliable delivery performance and patient acceptance features, particularly appealing in urban regions where healthcare quality and immunization coverage represent critical public health requirements.
Market expansion benefits from growing healthcare capabilities and pharmaceutical industry development that enable broader availability of needle-free injection systems for vaccination and therapeutic applications. Technology adoption follows patterns established in medical devices, where cost-effectiveness and clinical performance drive procurement decisions and operational deployment.
Market Intelligence Brief:
Germany establishes technology leadership through comprehensive healthcare quality programs and advanced medical technology infrastructure, integrating jet needle-free injectors across healthcare and pharmaceutical delivery applications. The country's 5.4% growth rate reflects established healthcare relationships and mature medical device adoption that supports widespread use of precision injection systems in hospitals and diabetes care facilities. Growth concentrates in major medical centers, including Munich, Berlin, and Hamburg, where healthcare excellence showcases mature device deployment that appeals to healthcare providers seeking proven delivery precision capabilities and patient safety applications.
German manufacturers leverage engineering expertise and comprehensive validation protocols, including clinical testing and performance documentation that create product credibility and technical advantages. The market benefits from established medical device standards and patient-centric care models that support injection system use while encouraging technology advancement and safety optimization.
Market Intelligence Brief:
Brazil's market expansion benefits from diverse healthcare demand, including hospital system development in São Paulo and Rio de Janeiro, vaccination program upgrades, and government healthcare initiatives that increasingly incorporate needle-free injectors for immunization applications. The country maintains a 4.9% growth rate, driven by rising healthcare activity and increasing recognition of patient safety technology benefits, including needle elimination and cross-contamination prevention.
Market dynamics focus on affordable injection systems that balance adequate performance with cost considerations important to Brazilian healthcare providers. Growing healthcare development creates continued demand for modern injection systems in new facility infrastructure and healthcare modernization projects.
Strategic Market Considerations:
The United States establishes market leadership through comprehensive diabetes care programs and advanced healthcare infrastructure development, integrating jet needle-free injectors across diabetes management and vaccination applications. The country's 4.5% growth rate reflects established pharmaceutical relationships and mature injection technology adoption that supports widespread use of needle-free systems in home care and clinical facilities. Growth concentrates in major healthcare markets, including major metropolitan areas and integrated health systems, where patient safety initiatives showcase mature device deployment that appeals to healthcare providers seeking proven safety capabilities and patient compliance applications.
American device manufacturers leverage established distribution networks and comprehensive support capabilities, including patient training programs and clinical evidence that create product acceptance and market advantages. The market benefits from mature regulatory standards and insurance reimbursement that support injection system use while encouraging technology advancement and therapeutic innovation.
Market Intelligence Brief:
The United Kingdom's healthcare technology market demonstrates established jet needle-free injector deployment with documented safety effectiveness in diabetes care applications and vaccination programs through integration with existing healthcare systems and patient safety infrastructure. The country maintains a 4.0% growth rate. Healthcare centers, including London, Manchester, and Birmingham, showcase quality installations where needle-free injectors integrate with comprehensive patient care platforms and electronic health systems to optimize treatment operations and safety effectiveness.
British healthcare providers prioritize patient safety and treatment adherence in injection system selection, creating demand for validated devices with proven features, including clinical effectiveness documentation and safety specifications. The market benefits from National Health Service infrastructure and patient-centric programs that provide consistent operational benefits and compliance with national healthcare standards.
Market Intelligence Brief:
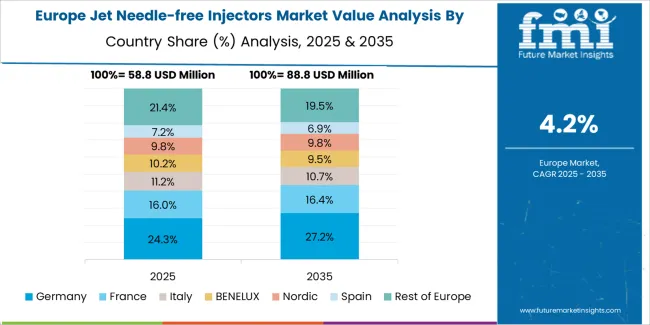
The jet needle-free injectors market in Europe is projected to grow from USD 82.9 million in 2025 to USD 196.4 million by 2035, registering a CAGR of 9.0% over the forecast period. Germany is expected to maintain its leadership position with a 33.7% market share in 2025, supported by its advanced healthcare infrastructure and major medical technology centers.
United Kingdom follows with a 22.8% share in 2025, driven by comprehensive patient safety programs and diabetes care initiatives. France holds a 18.5% share through vaccination program applications and pharmaceutical delivery requirements. Italy commands a 13.2% share, while Spain accounts for 11.8% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 6.4% to 7.1% by 2035, attributed to increasing device adoption in Nordic countries and emerging Eastern European healthcare facilities implementing patient safety modernization programs.
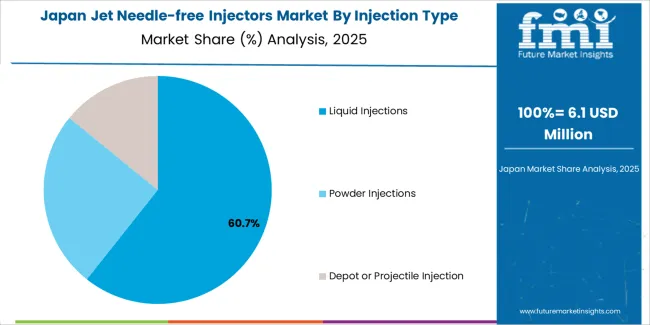
Japan demonstrates sophisticated jet needle-free injector deployment through premium healthcare infrastructure and advanced patient safety standards. The market maintains steady growth driven by diabetes management requirements and vaccination program modernization. Japanese manufacturers emphasize precision engineering and reliability features that meet rigorous medical device regulations. Technology partnerships with pharmaceutical companies enable specialized formulation development and delivery optimization suitable for Japanese healthcare priorities and demographic requirements including aging population care needs.
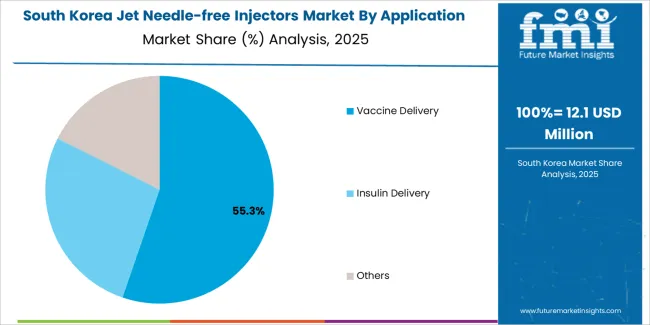
South Korea shows robust market expansion through intensive healthcare technology investment and medical device industry development programs. Seoul, major hospital systems, and diabetes care centers lead adoption with advanced needle-free injection systems incorporating digital tracking features. The market benefits from government healthcare initiatives and medical technology advancement programs that promote innovative delivery devices. Korean healthcare providers focus on patient convenience and treatment adherence features aligned with chronic disease management priorities and technological innovation objectives for future healthcare applications.
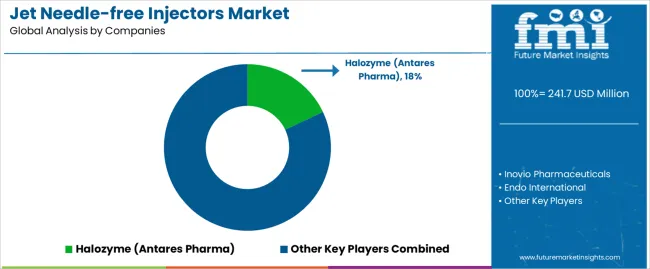
The market features approximately 15-20 specialized manufacturers, with the top 3-5 companies controlling roughly 65-70% of global revenue. Market leadership depends on pharmaceutical partnerships, clinical validation, and regulatory approval success rather than production scale advantages. Competition centers on delivery accuracy, device reliability, and formulation compatibility that drive adoption across healthcare institutions and patient populations.
Basic jet injection mechanisms and pressure delivery systems represent established technology, with manufacturers differentiating through dose precision, pharmaceutical compatibility range, and patient usability features. Margin opportunities exist in pharmaceutical collaborations offering drug-device combination products, proprietary cartridge systems, and integrated digital tracking that command premium positioning. Companies maintaining strong clinical evidence and pharmaceutical partnerships capture market advantages through product validation and therapeutic integration.
Global manufacturers leverage regulatory expertise for multi-regional approvals and pharmaceutical industry relationships, though specialized developers often compete effectively through innovative delivery mechanisms and targeted therapeutic applications. Technology innovation focuses on delivery pressure optimization, formulation compatibility enhancement, and digital connectivity integration rather than fundamental mechanism changes. Market dynamics favor participants with pharmaceutical expertise who understand drug delivery requirements and provide comprehensive clinical support alongside device offerings.
Pharmaceutical partnerships increasingly differentiate competitors, with leading manufacturers collaborating on drug-device combinations, formulation optimization, and therapeutic delivery systems that strengthen market positioning. Patient support programs and training services become important competitive factors. The market favors participants balancing device engineering with pharmaceutical collaboration effectiveness while maintaining product quality standards that meet regulatory requirements and healthcare performance expectations.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global platforms | Regulatory approvals, pharmaceutical partnerships, clinical validation | Proven safety, comprehensive evidence, multi-region availability | Innovation pace; therapeutic breadth |
| Technology innovators | Novel mechanisms; high-pressure systems; digital integration | Performance differentiation; patent protection; pharmaceutical interest | Market access; reimbursement challenges |
| Regional specialists | Local clinical relationships, regulatory knowledge, market access | Healthcare proximity; regional expertise; cost positioning | Technology advancement; scale limitations |
| Pharma-device ecosystems | Drug formulations, combination products, integrated solutions | Therapeutic validation; market access; prescription capture | Device manufacturing; technical service |
| Item | Value |
|---|---|
| Quantitative Units | USD 241.7 million |
| Injection Type | Powder Injections, Liquid Injections, Depot or Projectile Injection |
| Application | Vaccine Delivery, Insulin Delivery, Others |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, Middle East & Africa |
| Countries Covered | United States, China, Germany, India, United Kingdom, Japan, Brazil, France, Canada, South Korea, and 25+ additional countries |
| Key Companies Profiled | Halozyme (Antares Pharma), Inovio Pharmaceuticals, Endo International, Injex Pharma GmbH, National Medical Products (J-Tip), Recipharm, InsuJet, Miracle Medical, Medical International Technology (MIT) |
| Additional Attributes | Dollar sales by injection type and application categories, regional adoption trends across North America, East Asia, and Western Europe, competitive landscape with pharmaceutical device manufacturers and healthcare technology suppliers, healthcare provider preferences for delivery precision and patient safety, integration with pharmaceutical formulations and healthcare systems, innovations in injection technology and patient compliance enhancement, and development of connected delivery solutions with enhanced performance and healthcare optimization capabilities. |
The global jet needle-free injectors market is estimated to be valued at USD 241.7 million in 2025.
The market size for the jet needle-free injectors market is projected to reach USD 382.6 million by 2035.
The jet needle-free injectors market is expected to grow at a 4.7% CAGR between 2025 and 2035.
The key product types in jet needle-free injectors market are liquid injections, powder injections and depot or projectile injection.
In terms of application, vaccine delivery segment to command 55.0% share in the jet needle-free injectors market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Jet Mill Market Size and Share Forecast Outlook 2025 to 2035
Jetting Pumps Market
Inkjet Printers Market Size and Share Forecast Outlook 2025 to 2035
Inkjet Printer Market in Korea – Growth & Demand Forecast through 2035
Inkjet Coders Market Growth - Trends & Outlook 2025 to 2035
Key Players & Market Share in the Inkjet Printers Industry
Inkjet Paper Market Trends & Industry Growth Forecast 2024-2034
Inkjet Label Market Trends & Industry Growth Forecast 2024-2034
Gas Jet Compressor Market Size and Share Forecast Outlook 2025 to 2035
Steam Jet Ejector Market
Binder Jet Market
MEMS Inkjet Heads Market Growth – Trends and Forecast 2025-2035
Business Jet Market Size and Share Forecast Outlook 2025 to 2035
Japan Inkjet Printer Market - Industry Trends & Forecast 2025 to 2035
Thermal Inkjet Printer Market Size and Share Forecast Outlook 2025 to 2035
Thermal Inkjet Inks Market Size and Share Forecast Outlook 2025 to 2035
Examining Market Share Trends in the Thermal Inkjet Printer Industry
Thermal & Inkjet Disc Printers Market Analysis by Ribbon Type, Supplier, Technology, and Region Through 2035
Continuous Inkjet Inks Market Forecast and Outlook 2025 to 2035
Continuous Inkjet Printers Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA