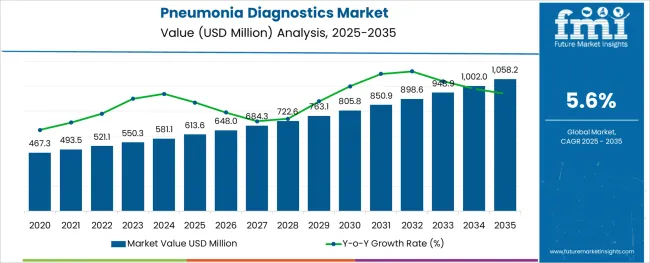
The Pneumonia Diagnostics Market is estimated to be valued at USD 613.6 million in 2025 and is projected to reach USD 1058.2 million by 2035, registering a compound annual growth rate (CAGR) of 5.6% over the forecast period.
The pneumonia diagnostics market is evolving rapidly due to heightened global focus on early disease detection, precision medicine, and outbreak preparedness. Increasing awareness of respiratory health, aging populations, and higher rates of hospital-acquired infections have intensified demand for efficient and reliable diagnostic methods.
Technological advancements in point-of-care testing, molecular assays, and biomarker identification have enabled faster and more accurate diagnosis, minimizing treatment delays. In both developed and emerging regions, healthcare providers are actively upgrading laboratory infrastructure to support syndromic testing and antimicrobial resistance tracking.
Additionally, the post-pandemic emphasis on respiratory pathogen surveillance has led to renewed investments in diagnostic platforms. Looking ahead, collaborations between biotech firms and public health agencies, along with regulatory fast-tracking of innovative tests, are expected to unlock new market opportunities across hospital and outpatient settings.
The market is segmented by Product Type and region. By Product Type, the market is divided into Streptococcus based, Legionella based, Chlamydophilla based, Mycoplasma Pneumonia based, and Viral Pneumonia based. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
The market is segmented by Product Type and region. By Product Type, the market is divided into Streptococcus based, Legionella based, Chlamydophilla based, Mycoplasma Pneumonia based, and Viral Pneumonia based. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
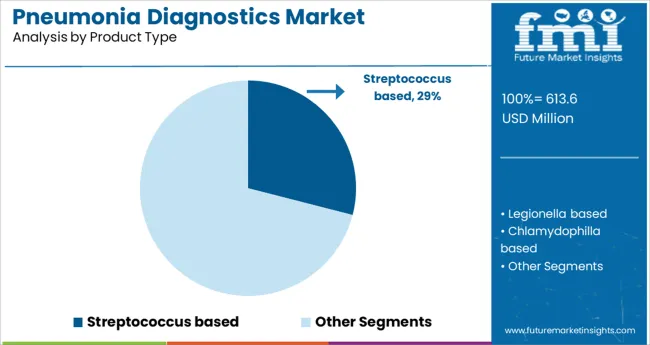
Streptococcus-based diagnostic solutions are projected to account for 29.0% of the total pneumonia diagnostics market revenue in 2025, establishing this product type as a leading segment. The prevalence of Streptococcus pneumoniae as a primary causative agent of community-acquired pneumonia has reinforced the need for targeted and rapid detection methods.
The segment's growth is being supported by widespread clinical reliance on antigen detection, PCR-based assays, and culture techniques specific to Streptococcus strains. Improved sensitivity of these diagnostic tools has allowed for early intervention, especially in vulnerable populations such as children and the elderly.
Furthermore, guidelines from health authorities emphasizing pathogen-specific diagnosis to combat antibiotic resistance have increased uptake. Continued innovation in portable and multiplexed devices further strengthens the segment’s position, as clinicians seek streamlined solutions for respiratory infection diagnosis in both acute and ambulatory care settings.
The overall sales in the pneumonia diagnostics market are projected to grow at 5.6% CAGR between 2025 and 2035, in comparison to the 4.5% CAGR registered from 2012 to 2024.
The rising incidence of pneumonia, the growing focus on early detection of infectious diseases, and advancements in diagnostics techniques are some of the key factors driving the global pneumonia diagnostic market.
Lung infections and other infectious diseases are becoming more common, there is an increase in the demand for diagnostics for pneumonia. One type of acute lung infection is pneumonia. A healthy person's lungs are made up of tiny sacs called alveoli that are filled with air as they breathe. Pneumonia causes the alveoli to bloat up with pus and fluid, which makes breathing challenging and limits oxygen intake.
The most common infectious cause of death in children worldwide is pneumonia. According to the World Health Organization 2024, pneumonia caused 740 to 180 deaths below the age of five in 2020, contributing to 14% of all deaths in children under the age of five but 22% of all deaths in children aged 1 to 5. Pneumonia affects children and families worldwide, although mortality is highest in Sub-Saharan Africa and South Asia.
The increasing adoption of point-of-care diagnostics and development in the detection of nucleic acid around the globe are set to boost the market in the given forecast period.
These factors, combined with the growing tendency toward preventive care are projected to increase the demand for pneumonia diagnostics products over the forecast period.
Rising Government Initiatives and Advancements in Diagnostic Technologies to Boost Market Over the Next Decade
The global pneumonia diagnostics market is likely to witness healthy growth owing to the increasing investments and initiatives by governments in emerging countries to modernize healthcare infrastructure.
The broad availability of rapid molecular diagnostic testing offers an unprecedented insight into pneumonia etiologies, challenging paradigms established by previous investigations from microbiological cultures.
Microscopy and culture of blood cultures, respiratory tract specimens, detection of antigens in urine and respiratory specimens, and detection of specific antibodies in the blood continue to be used in the routine laboratory evaluation of patients with pneumonia (serology). Nucleic acid detection technologies, such as polymerase chain reaction (PCR), have been available for more than two decades and are now commonplace in tertiary-level diagnostic laboratories.
Advancements in current diagnostics and innovation will thrive in the market in near future. For instance, Massachusetts Institute of Technology researchers have developed a sensor that can differentiate between viral and bacterial pneumonia infections, which they hope may aid doctors in selecting the best therapy.
The pricing pressure due to competition and difficulty in diagnosing can hamper the growth of the pneumonia diagnostics market during the forecast period.
In the community environment, the diagnosis and management of pneumonia usually become extremely complex. Physician approaches are primarily dependent on diagnosis information. In some circumstances, false positive results result in catastrophic patient mismanagement with antibiotics, as well as the possibility of superinfection and medication complications.
Immunoassay approaches for diagnosis are promising, however, they are still insufficient to screen for a wide range of pathogens. All these factors act as restraints in the growth of the pneumonia diagnostics market.
Similarly, low healthcare awareness across lower economies along with inadequate reimbursement policies is emerging as a significant impediment to the development of the pneumonia diagnostics market.
Easy Availability of Pneumonia Diagnostics Products Fuelling Sales in Canada
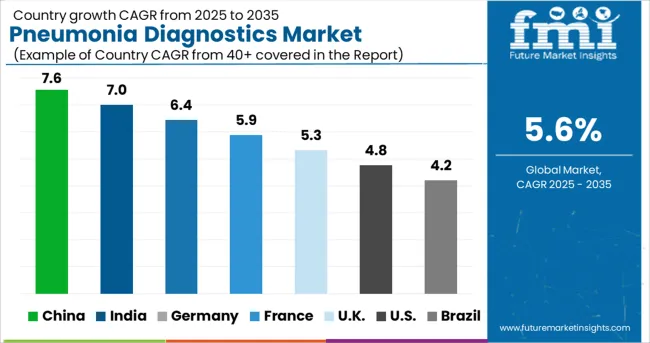
Canada held approximately 16.0% share in the North American pneumonia diagnostics market in 2024 and it is projected to display a growth rate of around 6.3% CAGR during the forecast period.
Growth in Canada’s pneumonia diagnostics market is projected to be driven by increasing production of pneumonia diagnostics products by top players, surging cases of pneumonia infection, and rising health awareness among people.
Favourable government support and the availability of better treatment approaches will further boost the market in the country over the next decade.
Increasing Incidence of Pneumonia and High Diagnostic Rates Driving Market in the USA
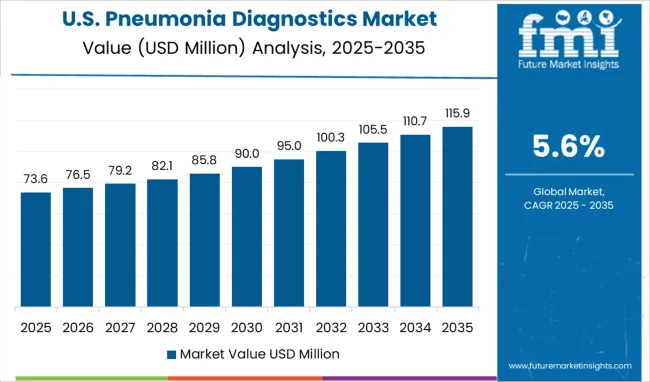
The USA dominated the North American pneumonia diagnostics market with a total market share of about 84.0% in 2024 and it is likely to expand at a steady pace during the forecast period.
Growth in the USA pneumonia diagnostics market is driven by the rising prevalence of pneumonia, increasing diagnostic screening rates due to high levels of awareness among people, and the presence of leading manufacturers.
Over the years, there has been a significant rise in the cases of pneumonia and pneumonia-related deaths across the USA which has necessitated the adoption of pneumonia diagnostics.
For instance, as per the Centers for Disease Control and Prevention (CDC), around 25.5% of USA adults suffered from pneumonia in 2024 while the total number of pneumonia-related deaths reached 47,601. In order to tackle this, people opt for various diagnostic tests and treatments. This will continue to boost the pneumonia diagnostics market in the country during the next ten years.
Similarly, increasing healthcare care spending and the presence of favourable reimbursement policies will further boost the demand for pneumonia diagnostic products in the USA market over the assessment period.
Continuous Advancements in Diagnostics Technologies to Boost Market in Germany
As per FMI, the overall pneumonia diagnostics market in Germany is set to exhibit a CAGR of nearly 7.5% during the forecast period (2025 to 2035). Germany is becoming increasingly popular when it comes to health and innovation.
With the increasing prevalence of chronic diseases and rising geriatric population demand for pneumonia diagnostics products will grow at a robust pace across Germany during the forecast period.
Similarly, the flourishing medical tourism sector and advancements in diagnostic technology will bode well for Germany’s pneumonia diagnostics market over the next ten years.
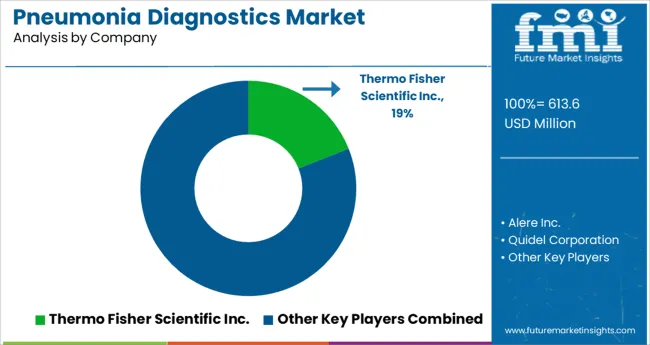
With several competitors in the pneumonia diagnostics production sphere, the overall market is fragmented. To meet consumer demand and expand their customer base, these companies are implementing methods such as mergers and acquisitions, partnerships and collaborations, and new product launches.
Instances of key developmental strategies by the industry players in the pneumonia diagnostics market are given below:
| Attribute | Details |
|---|---|
| Estimated Market Size (2025) | USD 613.6 million |
| Projected Market Size (2035) | USD 1058.2 million |
| Anticipated Growth Rate (2025 to 2035) | 5.6% |
| Forecast Period | 2025 to 2035 |
| Historical Data Available for | 2020 to 2024 |
| Market Analysis | USD Million for Value |
| Key Regions Covered | North America; Latin America; Europe; South Asia; East Asia; Oceania; and Middle East & Africa |
| Key Countries Covered | USA, Canada, Brazil, Mexico, Argentina, UK, Germany, Italy, Russia, Spain, France, BENELUX, Nordic Countries, India, Thailand, Indonesia, Malaysia, Philippines, Vietnam, Japan, China, South Korea, Australia, New Zealand, Israel, Turkey, GCC Countries, and South Africa |
| Key Market Segments Covered | Product and Region |
| Key Companies Profiled | Thermo Fisher Scientific Inc.; Alere Inc.; Quidel Corporation; Becton, Dickinson, and Company; Meridian Bioscience, Inc; Qiagen N.V.; bioMérieux SA; Bio-Rad Laboratories Inc.; Cardinal Health Inc.; Beckman Coulter Inc (DanaherCorporation); Hologic Inc.; La Roche Ltd.; Abbot Laboratories; Quest Diagnostics; Luminex Corporation |
| Report Coverage | Market Forecast, Company Share Analysis, Competition Intelligence, Drivers, Restraints, Opportunities and Threats Analysis, Market Dynamics and Challenges, and Strategic Growth Initiatives |
The global pneumonia diagnostics market is estimated to be valued at USD 613.6 USD million in 2025.
It is projected to reach USD 1,058.2 USD million by 2035.
The market is expected to grow at a 5.6% CAGR between 2025 and 2035.
The key product types are streptococcus based, legionella based, chlamydophilla based, mycoplasma pneumonia based and viral pneumonia based.
segment is expected to dominate with a 0.0% industry share in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Viral Pneumonia Treatment Market
Community-Acquired Bacterial Pneumonia Treatment Market Analysis by Drug Class, Dose Form, Route of Administration, Distribution Channel, and Region through 2035
DNA Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
HIV Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Food Diagnostics Services Market Size, Growth, and Forecast for 2025–2035
Rabies Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Cancer Diagnostics Market Analysis - Size, Share and Forecast 2025 to 2035
Tissue Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Sepsis Diagnostics Market Growth - Trends & Forecast 2025 to 2035
Poultry Diagnostics Market - Demand, Growth & Forecast 2025 to 2035
Protein Diagnostics Market Share, Size and Forecast 2025 to 2035
In-vitro Diagnostics Kit Market Size and Share Forecast Outlook 2025 to 2035
In Vitro Diagnostics Market Insights - Trends & Forecast 2025 to 2035
Clinical Diagnostics Market Insights – Size, Share & Forecast 2025 to 2035
Covid-19 Diagnostics Market – Demand, Growth & Forecast 2022-2032
In-Vitro Diagnostics Packaging Market
Connected Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Molecular Diagnostics In Pharmacogenomics Market Size and Share Forecast Outlook 2025 to 2035
Psychosis Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
The Companion Diagnostics Market is segmented by product, technology, application and end user from 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA