The cognitive diagnostics market is valued at USD 120.3 billion in 2025. As per FMI's analysis, the market will grow at a CAGR of 8.0% and reach USD 259.7 billion by 2035.
FMI analysis showed that the cognitive diagnostics or Cognitive Diagnostics industry made great progress in 2024, mainly due to quicker use of AI in neurodiagnostic tools and a stronger emphasis on finding diseases early. Companies have incorporated machine learning to enhance the precision of cognitive impairment diagnoses, further driving demand from healthcare providers. Additionally, FMI believes that regulatory approvals for AI-based diagnostics spurred adoption rates among hospitals and labs.
In 2024, the laboratory testing sector continued to be at the forefront, capturing a large share of the industry's revenue. FMI analysis indicates that significant investments concentrated on improving precision diagnostics, particularly in the detection of Alzheimer's and dementia. Furthermore, collaborations between tech companies and healthcare institutions led to AI-based tools going mainstream.
Looking forward to 2025 and beyond, FMI projects that rising investments in real-time cognitive testing and personalized medicine will fuel growth. Cloud-based cognitive testing solutions will further accelerate growth. With technology advancing to make diagnostics more accessible, the industry is likely to experience robust growth through 2035.
Industry Forecast Table
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 120.3 Billion |
| Industry Value (2035F) | USD 259.7 Billion |
| CAGR (2025 to 2035) | 8.0% |
The Cognitive Diagnostics industry is poised for steady growth, fuelled by AI-driven technologies and the growing demand for early diagnosis of neurological conditions. FMI research indicated that diagnostic labs and healthcare professionals will gain better accuracy and efficiency, while conventional diagnostic techniques stand to become obsolete. FMI believes that continued investment in precision medicine and cloud-based testing will continue to spur industry growth up to 2035.
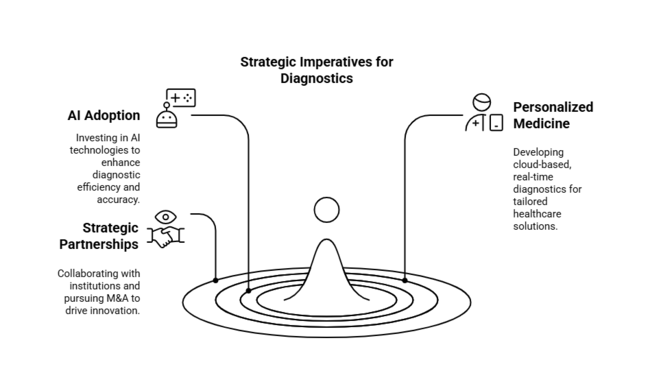
Accelerate AI-Driven Diagnostics Adoption
To improve efficiency and accuracy, we should first invest in AI-enabled Cognitive Diagnostics products. Partnering with technology companies and incorporating machine learning into current operations will give the competitive advantage.
Catch Up with Personalized Medicine Trends
Firms need to meet the growing demand for personalized cognitive testing by building cloud-based, real-time diagnostics. Being regulatory compliant and working with current healthcare systems will be essential for successful industry penetration.
Broaden Strategic Partnerships and M&A
Establishing strong collaborations with research institutions, healthcare organizations, and technology companies will drive innovation. CEOs need to consider mergers and acquisitions to centralize expertise, broaden service portfolios, and hasten the commercialization of future diagnostic technologies.
| Risk | Probability & Impact |
|---|---|
| Regulatory Challenges in AI Diagnostics | High Probability-High Impact |
| Data Privacy & Security Concerns | Medium Probability-High Impact |
| High Implementation Costs for Providers | Medium Probability-Medium Impact |
1-Year Executive Watchlist
| Priority | Immediate Action |
|---|---|
| AI-Powered Diagnostics Integration | Invest in R&D for machine learning-driven cognitive testing |
| Regulatory Compliance & Certification | Strengthen engagement with regulatory bodies for approvals |
| Expansion of Digital Testing Solutions | Develop and launch cloud-based cognitive assessment tools |
To stay ahead in the competition, executives need to accelerate AI integration in neurological diagnostics to remain ahead of changing healthcare needs. FMI research discovered that regulatory approvals are speeding up, providing an opportunity for early movers to establish dominance in AI-based precision testing.
Businesses need to invest in cloud-based solutions, regulatory expertise, and strategic collaborations with healthcare providers. FMI believes that early movers will set industry standards, while late movers will lose competitive advantage.
FMI analysis found that 84% of people involved worldwide considered accuracy in cognitive diagnostics important, especially for spotting neurodegenerative diseases like Alzheimer's and Parkinson's early on. AI automation posed a significant threat, with 78% citing advanced machine-learning technologies to enhance diagnostic precision.
Regional Differences
The regions are adopting AI-driven diagnostics
Divergent ROI Perceptions
While 74% of USA stakeholders reported strong ROI from AI-based diagnostics, only 42% of Japanese healthcare providers were convinced of cost savings, highlighting regional disparities in AI adoption economics
Consensus
Regional Preferences
Shared Challenges
90% of stakeholders identified the rising costs of AI deployment as a significant challenge, with software licensing expenses increasing by 25% year over year, further straining adoption efforts.
Regional Differences
Manufacturers
Distributors
According to FMI research, 76% of global diagnostic providers are looking to invest in AI-based cognitive testing in the next five years.
Regional Focus Areas
Regulatory Impact
Strategic Insights
According to the FMI, companies should adapt their AI solutions according to the region; high-performance models would be preferred by companies in the USA, while regulatory compliance with AI in Western Europe and cost-effective solutions in Japan and South Korea. Players across industry verticals need to match local industry realities to attain optimum adoption and commercial success until 2035.
| Countries | Key Regulations & Certifications |
|---|---|
| United States | The FDA regulates AI-based Cognitive Diagnostics products, requiring them to get either 510(k) clearance, De Novo approval, or Pre- Industry Approval (PMA) before they can be sold. HIPAA imposes strict data security and privacy standards for AI-based diagnostics, keeping patient data safe. The Centers for Medicare & Medicaid Services (CMS) established rules for reimbursement under which AI products would be eligible for insurance coverage. Moreover, certain states, like California, mandate AI-specific health regulations aimed at transparency and reducing bias. |
| United Kingdom | AI-powered diagnostics as a medical device must comply with regulatory requirements established by the Medicines and Healthcare Products Regulatory Agency (MHRA). The UK GDPR legislation mandates severe privacy and security protocols for AI models processing patient information. NHS Digital has to ensure that AI tools used for diagnosing patients follow strict buying and security rules before they can be used in the public health system. The AI and Digital Health Regulation Act of 2024 requires that AI systems used in healthcare must be clear about how they work and must work to remove any bias in medical decisions. |
| France | Clinical environments require the deployment of AI-based neurological diagnostics with Haute Autorité de Santé (HAS) certification. The Commission Nationale de l'Informatique et des Libertés (CNIL) applies the discipline of AI ethics and patient data protection regulations. AI-based diagnostic devices marketed in France must comply with the EU Medical Device Regulation (MDR). The government also introduced an AI-Specific Reimbursement Pilot Program to assess how AI-based diagnostics can be incorporated into the national healthcare reimbursement regime. |
| Germany | AI diagnostic devices need to be approved by the Federal Institute for Drugs and Medical Devices (BfArM) according to strict clinical validation regulations. The DiGA Fast Track Process allows AI-based neurological diagnostics to be reimbursed within the German healthcare system. The EU Medical Device Regulation (MDR) and GDPR need to be complied with by AI-based medical solutions. The KI- Strategie (AI Strategy 2024) promotes the use of AI but also requires explainability and bias audits for healthcare use. |
| Italy | The Ministry of Health and the Italian Medicines Agency (AIFA) regulate AI-based Cognitive Diagnostics approvals. The healthcare system requires all AI-based medical diagnostics to comply with EU MDR before implementing them. Certain Italian regions enforce additional independent AI adoption policies, resulting in varying regulatory requirements across the nation. The AI & Digital Transformation Plan 2025 will ensure AI integration in public healthcare with additional validation requirements. |
| South Korea | The Ministry of Food and Drug Safety (MFDS) requires that AI Cognitive Diagnostics products undergo thorough clinical testing to ensure they are safe and effective. The Digital Health Sandbox Program provides expedited approval for AI diagnostics, promoting innovation without compromising safety. South Korea's K-Data Law mandates strict patient data protection regulations for AI model training. The government provides incentives for AI adoption, but firms must navigate complex regulatory approval processes. |
| Japan | The Pharmaceuticals and Medical Devices Agency (PMDA) allows AI-based cognitive diagnostics, but only after requiring thorough clinical testing before they can be sold. The Japan Agency for Medical Research and Development (AMED) funds AI-driven cognitive health programs but has strict performance requirements. Japan's regulatory environment has stringent AI testing requirements, which makes it one of the most difficult scenario s for the deployment of rapid AI healthcare. |
| China | AI-based neurological diagnostics need approval from the National Medical Products Administration (NMPA). The cybersecurity law requires AI diagnostic devices that hold patient information to store it locally, preventing cross-border data flow. Investment from the government in the Healthy China 2030 strategy has accelerated the development of AI-based healthcare, yet regulatory barriers remain significant, especially regarding foreign AI medical devices' access to the Chinese market. |
| Australia & New Zealand | The Therapeutic Goods Administration (TGA) in Australia regulates AI Cognitive Diagnostics tools in conformity with the Medical Device Regulatory Framework. Medsafe in New Zealand regulates AI-based medical devices with stringent compliance controls. AI diagnosis tools are required to comply with the privacy requirements of both nations' Data Protection Acts. Healthcare promotes the adoption of AI, yet compelled clinical validation remains a regulatory norm. |
| India | The Central Drugs Standard Control Organization (CDSCO) regulates Cognitive Diagnostics AI-based tools in India under the Medical Device Rules. India is yet to establish regulations for mainstream AI in healthcare, but the Digital Personal Data Protection Act is enough to ensure patient data protection for AI models. Approval from the Health Ministry is needed for AI-driven diagnostics before their use in clinical environments, and India's move toward AI implementation in healthcare is slowly establishing its regulatory environment. |
Positron Emission Tomography (PET) and PET/CT are projected to lead the cognitive diagnostics industry with a robust CAGR of 9.2% from 2025 to 2035. This growth is primarily driven by the increasing adoption of PET scans for early detection of Alzheimer's disease, dementia, and other cognitive disorders. As healthcare providers continue to prioritize early diagnosis and personalized treatment plans, PET’s ability to detect subtle changes in brain activity and structure at the molecular level is crucial.
Furthermore, advancements in hybrid PET/CT technology, which combines anatomical and functional imaging, are enhancing the diagnostic accuracy, making PET a key tool in precision medicine. This segment is expected to see significant investments in the coming years, solidifying its position as a cornerstone in neurodiagnostics.
In addition to its growing diagnostic potential, PET imaging is gaining prominence due to its high sensitivity and non-invasive nature. The segment is witnessing rising demand as hospitals and diagnostic centers strive to improve the accuracy of cognitive impairment diagnoses, particularly for Alzheimer's and other neurodegenerative diseases.
Mild Cognitive Impairment (MCI) is rapidly emerging as the fastest growing indication in cognitive diagnostics, boasting a projected CAGR of 8.2% from 2025 to 2035. This growth is propelled by the increasing recognition of MCI as a precursor to Alzheimer’s and other forms of dementia. As the global aging population rises, the demand for early detection of MCI becomes more critical to mitigating long-term healthcare burdens.
With advancements in diagnostic technologies such as imaging, biomarker testing, and neuropsychological evaluations, MCI is now more identifiable and treatable. The focus on early intervention to slow or prevent disease progression will drive continued expansion for this indication.
The identification and management of MCI are becoming central to the treatment of Alzheimer’s and dementia, creating significant opportunities for healthcare providers. The industry for diagnostic tools that can accurately assess MCI is rapidly evolving, with key players in the neurodiagnostic space innovating to meet this growing need. As such, MCI diagnostics are poised to take a leading position in cognitive healthcare, attracting increased investments.
Home care settings are projected to become the fastest growing end user segment in cognitive diagnostics, with a CAGR of 8.7% from 2025 to 2035. The growth in this segment reflects a broader shift towards decentralized healthcare, where individuals are seeking more accessible and flexible diagnostic solutions outside traditional clinical settings.
The rise of home-based cognitive screening tools, such as digital assessments and remote monitoring technologies, has revolutionized the ability to conduct early-stage cognitive impairment tests at home. This is particularly valuable for aging populations who may have limited access to healthcare facilities. As technology advances, home care settings are becoming an essential part of the healthcare ecosystem.
The growing preference for home care diagnostics is further fueled by the desire for cost-effective, non-invasive, and user-friendly solutions. Rapid home screening tests are gaining traction, allowing patients and caregivers to monitor cognitive health regularly. This trend is expected to result in a paradigm shift in cognitive diagnostics, with more patients opting for home-based solutions as part of a wider effort to reduce healthcare costs.
The cognitive diagnostics industry in the USA is expected to grow at a CAGR of 8.4% from 2025 to 2035. Due to sophisticated healthcare infrastructure, substantial R&D investment, and high penetration rates of AI-based diagnostic solutions, in this space, United States acts as a industry leader. The USA. dominates the North American region with a total industry share of around 26.8%.
AI-based diagnostics for high-risk devices are getting a new set of rules from the FDA, allowing approvals for devices that use machine learning to assess cognitive function. Driving Factors: Aging populations & rising prevalence of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Additionally, reimbursement policies from CMS and private payers are expanding access to neurological diagnostics by hospitals and clinics.
The cognitive diagnostics industry in the United Kingdom from 2025 to 2035 is expected to grow at a CAGR of 7.9%. The UK government has actively backed the use of AI-based neurological diagnostics in their National Health Service (NHS) by providing research funding and speeding up the approval of digital health solutions.
The MHRA governs AI-powered diagnostic devices, and firms must comply with rigorous data privacy legislation under UK GDPR. Increasing awareness on early detection of dementia and cognitive impairments is anticipated to spur the growth of these technologies. However, constraints on NHS budgets and the slow adoption of public health care can limit industry expansion.
Forecasts indicate a CAGR of 7.5% for the Cognitive Diagnostics industry in France between 2025 and 2035. Strong government support and incentives for research have positioned the nation at the forefront of AI-based healthcare innovation in Europe. The nation has achieved quality certification for AI-driven neurological diagnostics, adhering to high standards of clinical validation.
France’s graying population and rising burden of neurodegenerative diseases like Alzheimer’s are driving demand. The EU's own Medical Device Regulation (MDR) adds further regulatory complexity, but France's focus on health care AI and its National Strategy for AI incentivize growth.
The cognitive diagnostics industry in Germany is expected to grow from 2025 to 2035 with a CAGR of 8.2%. The strong healthcare system, high use of digital health solutions, and the quick approval process for digital health apps help create a well-developed industry for neurological diagnostics in Germany. Germany holds a share of 5.1% in the global industry.
A broad set of rules with strict approvals makes sure that AI-based neurological diagnostics are safe and effective. Aging demographics and the increasing incidence of dementia at a national level help propel growth, along with the country's efforts to adopt AI in healthcare. The private insurance sector in Germany is facilitating adoption as well.
The Italy Cognitive Diagnostics industry is anticipated to grow at a CAGR of 7.1% from 2025 to 2035. An aging population and an increasing focus on AI in healthcare are driving the industry's slow but steady evolution. Italy is slowly addressing the early diagnosis of cognitive disorders by implementing AI-based diagnostics in the public healthcare system, which is under the Ministry of Health (Servizio Sanitario Nazionale-SSN).
However, regional variations in healthcare infrastructure and slow adoption of digital health technologies add to potential challenges. AI-driven diagnostic tools also need to comply with the EU’s MDR requirements, which can create regulatory hurdles for industry access.
The industry in South Korea is expected to grow at a CAGR of 8.5% during the period 2025 to 2035. Strong government support for digital healthcare innovation has already made South Korea a leader in AI-enabled medical care. The initiative is being supported by the Ministry of Food and Drug Safety (MFDS), which has created channels for the accelerated approval of AI-enabled diagnostics, resulting in broad adoption throughout the nation.
South Korea's aging population and high prevalence of cognitive diseases in the country are bolstering demand for early diagnosis. In South Korea, the Digital Health Sandbox Program accelerates commercialization of the AI-based diagnostic solution, making it one of the fastest-growing countries in the neurological diagnostics market.
The industry in Japan is expected to be 7.8% CAGR between 2025 and 2035. Japan's super-aging population is driving an increasing prevalence of dementia and similar conditions, thereby heightening the need for neurological diagnostics. The Pharmaceuticals and Medical Devices Agency (PMDA) in Japan rigorously regulates AI-based Cognitive Diagnostics solutions.
Japan is heavily investing in AI healthcare innovation, but its adoption is slow due to concerns about high prices and over-engineering. AI programs for healthcare-driven by government and reimbursement policies, as well as cognitive testing-will drive acceleration, particularly in the hospitals and nursing homes.
China’s sale is anticipated to record a value of USD9.46 billion by 2030. With government-backed campaigns such as Healthy China 2030, China is emerging as a significant industry in AI-enabled healthcare. China has a market share of 9.5% in the global industry. The National Medical Products Administration (NMPA) regulates AI-powered neurological diagnostics and ensures that devices comply with domestic medical device regulations.
A rapidly growing, aging population with increasing cognitive disorders is driving the demand for early detection solutions. But strict data localization laws in China make entry for global AI players exceptionally tough: localized AI must be trained, and cybersecurity regulations must be followed.
The industry in India is estimated to grow at a compound annual growth rate of 8.7% between 2025 and 2035.India holds a share of around 6.4% in the global industry. Factors such as increasing healthcare digitization, rising awareness about early diagnoses of cognitive disorders, and increasing private healthcare expenditures are expected to drive the industry during the forecast period.
Though AI-based diagnostics are regulated within the purview of the Medical Device Rules of the Central Drugs Standard Control Organization (CDSCO), India is still shifting toward a comprehensive AI healthcare framework. Government initiatives like the NDHM are accelerating AI adoption, but challenges remain in terms of affordability and access. The private sector is leading the growth in numbers.
Several leading companies in the cognitive diagnostics sector compete through innovation, pricing strategies, and strategic partnerships. Companies are investing in AI-based diagnostic solutions, biomarker-based testing, and advances in neuroimaging to gain a competitive edge. Pricing is a differentiator, with companies having tiered offers for hospitals, clinics, and home consumption.
They plan to grow by entering emerging markets and partnering with research institutions. Mergers and acquisitions drive technological integration, while partnerships with pharmaceutical companies enhance diagnostic precision. Leading companies are heavily investing in research and development (R&D) for early-stage Alzheimer's and dementia diagnostics, driving industry expansion. Competition intensity is steep, inducing ceaseless development and globalization.
Industry Share Breakdown
IBM (IBM Watson Health)
Google Health (DeepMind, Alphabet Inc.)
Microsoft (Nuance Communications, Azure AI)
NVIDIA (Clara AI)
Siemens Healthineers
GE Healthcare
The industry is segmented intobrain imaging tests, magnetic resonance imaging (MRI), computerized tomography (CT), positron emission tomography (PET) and PET/CT, laboratory testing, cerebrospinal fluid (CSF), blood tests, mental status testing, mini-mental state exam (MMSE), Montreal cognitive assessment (MoCA), rapid home screening tests, neuropsychological testing, electroencephalogram (EEG), cognitive function & behavioral tests, neuropsychiatric inventory questionnaire (NPI-Q).
The industry is segmented intoalzheimer’s disease, attention deficit/hyperactivity disorder, dementia, epilepsy-related cognitive dysfunction, mild cognitive impairment, parkinson’s disease-related cognitive dysfunction, stroke-related cognitive dysfunction, traumatic brain injury, others.
The industry is fragmented intohospitals, neurology clinics, cognitive behavioral therapy centers, rehabilitation centers, academic and research institutes, diagnostic imaging centers, home care settings.
The industry is studied acrossNorth America, Latin America, Europe, South Asia, East Asia, Oceania, The Middle East and Africa (MEA).
The rise in neurological disorders, advancements in AI-based diagnostic technology, and increased use of brain imaging technologies are driving the sector.
Growing healthcare investments, early disease detection programs, and advancements in diagnostic imaging and lab testing will all contribute to strong growth.
Major players are Cognetivity Neurosciences, Cognivue, Inc., Google Health (DeepMind, Alphabet Inc.), Cogstate Ltd., Cambridge Cognition Ltd., IBM (IBM Watson Health), Diadem srl, NVIDIA (Clara AI), C₂N Diagnostics, Siemens Healthineers, Roche Diagnostics, Abbott Laboratories, GE Healthcare, Janssen Pharmaceuticals, and Biogen Inc.
Brain imaging tests, especially MRI and PET scans, are likely to see maximum growth as they are most effective in early-stage neurological tests.
The industry is projected to reach USD 259.7 billion by 2035, driven by increasing adoption in hospitals, neurology clinics, and home care settings.
Table 01: Global Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 02: Global Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 03: Global Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 04: Global Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 05: Global Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 06: Global Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 07: Global Market Size (US$ Million) Analysis 2012 to 2022, By Region
Table 08: Global Market Size (US$ Million) Forecast 2023 to 2033, By Region
Table 09: North America Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 10: North America Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 11: North America Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 12: North America Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 13: North America Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 14: North America Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 15: North America Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 16: North America Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 17: Latin America Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 18: Latin America Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 19: Latin America Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 20: Latin America Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 21: Latin America Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 22: Latin America Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 23: Latin America Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 24: Latin America Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 25: Europe Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 26: Europe Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 27: Europe Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 28: Europe Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 29: Europe Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 30: Europe Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 31: Europe Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 32: Europe Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 33: South Asia Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 34: South Asia Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 35: South Asia Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 36: South Asia Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 37: South Asia Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 38: South Asia Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 39: South Asia Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 40: South Asia Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 41: East Asia Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 42: East Asia Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 43: East Asia Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 44: East Asia Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 45: East Asia Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 46: East Asia Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 47: East Asia Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 48: East Asia Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 49: Oceania Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 50: Oceania Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 51: Oceania Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 52: Oceania Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 53: Oceania Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 54: Oceania Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 55: Oceania Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 56: Oceania Market Size (US$ Million) Forecast 2023 to 2033, By End User
Table 57: Middle East & Africa Market Size (US$ Million) Analysis 2012 to 2022, By Country
Table 58: Middle East & Africa Market Size (US$ Million) Forecast 2023 to 2033, By Country
Table 59: Middle East & Africa Market Size (US$ Million) Analysis 2012 to 2022, By Diagnosis
Table 60: Middle East & Africa Market Size (US$ Million) Forecast 2023 to 2033, By Diagnosis
Table 61: Middle East & Africa Market Size (US$ Million) Analysis 2012 to 2022, By Indication
Table 62: Middle East & Africa Market Size (US$ Million) Forecast 2023 to 2033, By Indication
Table 63: Middle East & Africa Market Size (US$ Million) Analysis 2012 to 2022, By End User
Table 64: Middle East & Africa Market Size (US$ Million) Forecast 2023 to 2033, By End User
Figure 01: Global Market Split By Region, 2023 (E)
Figure 02: Global Market Split By Diagnosis, 2023 (E)
Figure 03: Global Market Split By Indication, 2023 (E)
Figure 04: Global Market Split By End User, 2023 (E)
Figure 05: Global Neurology Services Market Overview
Figure 06: Global Market - COVID-19 Impact, 2017 to 2033
Figure 07: Global Market Size (US$ Million) Analysis, 2012 to 2022
Figure 08: Global Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 09: Global Market Absolute $ Opportunity Analysis, 2022 to 2033
Figure 10: Global Market Analysis By Diagnosis –2023 & 2033
Figure 11: Global Market Y-o-Y Growth Projections By Diagnosis, 2023 to 2033
Figure 12: Global Market Attractiveness, By Diagnosis, 2023- 2033
Figure 13: Global Market Analysis By Indication –2023 & 2033
Figure 14: Global Market Y-o-Y Growth Projections By Indication, 2023 to 2033
Figure 15: Global Market Attractiveness, By Indication, 2023- 2033
Figure 16: Global Market Analysis By End User –2023 & 2033
Figure 17: Global Market Y-o-Y Growth Projections By Indication, 2023 to 2033
Figure 18: Global Market Attractiveness, By End User, 2023- 2033
Figure 19: Global Market Analysis by Region–2023 & 2033
Figure 20: Global Market Y-o-Y Growth Projections by Region, 2023 to 2033
Figure 21: Global Market Attractiveness, By Region, 2023 to 2033
Figure 22: North America Market Value Share, by Diagnosis, 2023 (E)
Figure 23: North America Market Value Share, by Indication, 2023 (E)
Figure 24: North America Market Value Share, by End User, 2023 (E)
Figure 25: North America Market Value Share, by Country, 2023 (E)
Figure 26: North America Market Size (US$ Million) Analysis, 2012 to 2022
Figure 27: North America Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 28: North America Market Attractiveness Analysis, by Country
Figure 29: North America Market Attractiveness Analysis, By Diagnosis
Figure 30: North America Market Attractiveness Analysis, by Indication
Figure 31: North America Market Attractiveness Analysis, By End User
Figure 32: USA Market Value Proportion Analysis, 2022
Figure 33: Global Vs. USA Growth Comparison, 2022 to 2033
Figure 34: USA Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 35: USA Market Share Analysis (%), By Indication, 2022 & 2033
Figure 36: USA Market Share Analysis (%), By End User, 2022 & 2033
Figure 37: Canada Market Value Proportion Analysis, 2022
Figure 38: Global Vs. Canada Growth Comparison, 2022 to 2033
Figure 39: Canada Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 40: Canada Market Share Analysis (%), By Indication, 2022 & 2033
Figure 41: Canada Market Share Analysis (%), By End User, 2022 & 2033
Figure 42: Latin America Market Value Share, by Diagnosis, 2023 (E)
Figure 43: Latin America Market Value Share, by Indication, 2023 (E)
Figure 44: Latin America Market Value Share, by End User, 2023 (E)
Figure 45: Latin America Market Value Share, by Country, 2023 (E)
Figure 46: Latin America Market Size (US$ Million) Analysis, 2012 to 2022
Figure 47: Latin America Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 48: Latin America Market Attractiveness Analysis, by Country
Figure 49: Latin America Market Attractiveness Analysis, By Diagnosis
Figure 50: Latin America Market Attractiveness Analysis, by Indication
Figure 51: Latin America Market Attractiveness Analysis, By End User
Figure 52: Brazil Market Value Proportion Analysis, 2022
Figure 53: Global Vs. Brazil Growth Comparison, 2022 to 2033
Figure 54: Brazil Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 55: Brazil Market Share Analysis (%), By Indication, 2022 & 2033
Figure 56: Brazil Market Share Analysis (%), By End User, 2022 & 2033
Figure 57: Mexico Market Value Proportion Analysis, 2022
Figure 58: Global Vs. Mexico Growth Comparison, 2022 to 2033
Figure 59: Mexico Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 60: Mexico Market Share Analysis (%), By Indication, 2022 & 2033
Figure 61: Mexico Market Share Analysis (%), By End User, 2022 & 2033
Figure 62: Argentina Market Value Proportion Analysis, 2022
Figure 63: Global Vs. Argentina Growth Comparison, 2022 to 2033
Figure 64: Argentina Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 65: Argentina Market Share Analysis (%), By Indication, 2022 & 2033
Figure 66: Argentina Market Share Analysis (%), By End User, 2022 & 2033
Figure 67: Europe Market Value Share, by Diagnosis, 2023 (E)
Figure 68: Europe Market Value Share, by Indication, 2023 (E)
Figure 69: Europe Market Value Share, by End User, 2023 (E)
Figure 70: Europe Market Value Share, by Country, 2023 (E)
Figure 71: Europe Market Size (US$ Million) Analysis, 2012 to 2022
Figure 72: Europe Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 73: Europe Market Attractiveness Analysis, by Country
Figure 74: Europe Market Attractiveness Analysis, By Diagnosis
Figure 75: Europe Market Attractiveness Analysis, by Indication
Figure 76: Europe Market Attractiveness Analysis, By End User
Figure 77: Germany Market Value Proportion Analysis, 2022
Figure 78: Global Vs. Germany Growth Comparison, 2022 to 2033
Figure 79: Germany Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 80: Germany Market Share Analysis (%), By Indication, 2022 & 2033
Figure 81: Germany Market Share Analysis (%), By End User, 2022 & 2033
Figure 82: Italy Market Value Proportion Analysis, 2022
Figure 83: Global Vs. Italy Growth Comparison, 2022 to 2033
Figure 84: Italy Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 85: Italy Market Share Analysis (%), By Indication, 2022 & 2033
Figure 86: Italy Market Share Analysis (%), By End User, 2022 & 2033
Figure 87: France Market Value Proportion Analysis, 2022
Figure 88: Global Vs. France Growth Comparison, 2022 to 2033
Figure 89: France Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 90: France Market Share Analysis (%), By Indication, 2022 & 2033
Figure 91: France Market Share Analysis (%), By End User, 2022 & 2033
Figure 92: United kingdom Market Value Proportion Analysis, 2022
Figure 93: Global Vs. United kingdom Growth Comparison, 2022 to 2033
Figure 94: United kingdom Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 95: United kingdom Market Share Analysis (%), By Indication, 2022 & 2033
Figure 96: United kingdom Market Share Analysis (%), By End User, 2022 & 2033
Figure 97: Spain Market Value Proportion Analysis, 2022
Figure 98: Global Vs. Spain Growth Comparison, 2022 to 2033
Figure 99: Spain Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 100: Spain Market Share Analysis (%), By Indication, 2022 & 2033
Figure 101: Spain Market Share Analysis (%), By End User, 2022 & 2033
Figure 102: BENELUX Market Value Proportion Analysis, 2022
Figure 103: Global Vs. BENELUX Growth Comparison, 2022 to 2033
Figure 104: BENELUX Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 105: BENELUX Market Share Analysis (%), By Indication, 2022 & 2033
Figure 106: BENELUX Market Share Analysis (%), By End User, 2022 & 2033
Figure 107: Nordic Countries Market Value Proportion Analysis, 2022
Figure 108: Global Vs. Nordic Countries Growth Comparison, 2022 to 2033
Figure 109: Nordic Countries Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 110: Nordic Countries Market Share Analysis (%), By Indication, 2022 & 2033
Figure 111: Nordic Countries Market Share Analysis (%), By End User, 2022 & 2033
Figure 112: Russia Market Value Proportion Analysis, 2022
Figure 113: Global Vs. Russia Growth Comparison, 2022 to 2033
Figure 114: Russia Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 115: Russia Market Share Analysis (%), By Indication, 2022 & 2033
Figure 116: Russia Market Share Analysis (%), By End User, 2022 & 2033
Figure 117: South Asia Market Value Share, by Diagnosis, 2023 (E)
Figure 118: South Asia Market Value Share, by Indication, 2023 (E)
Figure 119: South Asia Market Value Share, by End User, 2023 (E)
Figure 120: South Asia Market Value Share, by Country, 2023 (E)
Figure 121: South Asia Market Size (US$ Million) Analysis, 2012 to 2022
Figure 122: South Asia Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 123: South Asia Market Attractiveness Analysis, by Country
Figure 124: South Asia Market Attractiveness Analysis, By Diagnosis
Figure 125: South Asia Market Attractiveness Analysis, by Indication
Figure 126: South Asia Market Attractiveness Analysis, By End User
Figure 127: India Market Value Proportion Analysis, 2022
Figure 128: Global Vs. India Growth Comparison, 2022 to 2033
Figure 129: India Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 130: India Market Share Analysis (%), By Indication, 2022 & 2033
Figure 131: India Market Share Analysis (%), By End User, 2022 & 2033
Figure 132: Thailand Market Value Proportion Analysis, 2022
Figure 133: Global Vs. Thailand Growth Comparison, 2022 to 2033
Figure 134: Thailand Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 135: Thailand Market Share Analysis (%), By Indication, 2022 & 2033
Figure 136: Thailand Market Share Analysis (%), By End User, 2022 & 2033
Figure 137: Malaysia Market Value Proportion Analysis, 2022
Figure 138: Global Vs. Malaysia Growth Comparison, 2022 to 2033
Figure 139: Malaysia Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 140: Malaysia Market Share Analysis (%), By Indication, 2022 & 2033
Figure 141: Malaysia Market Share Analysis (%), By End User, 2022 & 2033
Figure 142: Philippines Market Value Proportion Analysis, 2022
Figure 143: Global Vs. Philippines Growth Comparison, 2022 to 2033
Figure 144: Philippines Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 145: Philippines Market Share Analysis (%), By Indication, 2022 & 2033
Figure 146: Philippines Market Share Analysis (%), By End User, 2022 & 2033
Figure 147: Indonesia Market Value Proportion Analysis, 2022
Figure 148: Global Vs. Indonesia Growth Comparison, 2022 to 2033
Figure 149: Indonesia Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 150: Indonesia Market Share Analysis (%), By Indication, 2022 & 2033
Figure 151: Indonesia Market Share Analysis (%), By End User, 2022 & 2033
Figure 152: Vietnam Market Value Proportion Analysis, 2022
Figure 153: Global Vs. Vietnam Growth Comparison, 2022 to 2033
Figure 154: Vietnam Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 155: Vietnam Market Share Analysis (%), By Indication, 2022 & 2033
Figure 156: Vietnam Market Share Analysis (%), By End User, 2022 & 2033
Figure 157: East Asia Market Value Share, by Diagnosis, 2023 (E)
Figure 158: East Asia Market Value Share, by Indication, 2023 (E)
Figure 159: East Asia Market Value Share, by End User, 2023 (E)
Figure 160: East Asia Market Value Share, by Country, 2023 (E)
Figure 161: East Asia Market Size (US$ Million) Analysis, 2012 to 2022
Figure 162: East Asia Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 163: East Asia Market Attractiveness Analysis, by Country
Figure 164: East Asia Market Attractiveness Analysis, By Diagnosis
Figure 165: East Asia Market Attractiveness Analysis, by Indication
Figure 166: East Asia Market Attractiveness Analysis, By End User
Figure 167: China Market Value Proportion Analysis, 2022
Figure 168: Global Vs. China Growth Comparison, 2022 to 2033
Figure 169: China Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 170: China Market Share Analysis (%), By Indication, 2022 & 2033
Figure 171: China Market Share Analysis (%), By End User, 2022 & 2033
Figure 172: Japan Market Value Proportion Analysis, 2022
Figure 173: Global Vs. Japan Growth Comparison, 2022 to 2033
Figure 174: Japan Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 175: Japan Market Share Analysis (%), By Indication, 2022 & 2033
Figure 176: Japan Market Share Analysis (%), By End User, 2022 & 2033
Figure 177: South Korea Market Value Proportion Analysis, 2022
Figure 178: Global Vs. South Korea Growth Comparison, 2022 to 2033
Figure 179: South Korea Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 180: South Korea Market Share Analysis (%), By Indication, 2022 & 2033
Figure 181: South Korea Market Share Analysis (%), By End User, 2022 & 2033
Figure 182: Oceania Market Value Share, by Diagnosis, 2023 (E)
Figure 183: Oceania Market Value Share, by Indication, 2023 (E)
Figure 184: Oceania Market Value Share, by End User, 2023 (E)
Figure 185: Oceania Market Value Share, by Country, 2023 (E)
Figure 186: Oceania Market Size (US$ Million) Analysis, 2012 to 2022
Figure 187: Oceania Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 188: Oceania Market Attractiveness Analysis, by Country
Figure 189: Oceania Market Attractiveness Analysis, By Diagnosis
Figure 190: Oceania Market Attractiveness Analysis, by Indication
Figure 191: Oceania Market Attractiveness Analysis, By End User
Figure 192: Australia Market Value Proportion Analysis, 2022
Figure 193: Global Vs. Australia Growth Comparison, 2022 to 2033
Figure 194: Australia Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 195: Australia Market Share Analysis (%), By Indication, 2022 & 2033
Figure 196: Australia Market Share Analysis (%), By End User, 2022 & 2033
Figure 197: New Zealand Market Value Proportion Analysis, 2022
Figure 198: Global Vs. New Zealand Growth Comparison, 2022 to 2033
Figure 199: New Zealand Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 200: New Zealand Market Share Analysis (%), By Indication, 2022 & 2033
Figure 201: New Zealand Market Share Analysis (%), By End User, 2022 & 2033
Figure 202: Middle East & Africa Market Value Share, by Diagnosis, 2023 (E)
Figure 203: Middle East & Africa Market Value Share, by Indication, 2023 (E)
Figure 204: Middle East & Africa Market Value Share, by End User, 2023 (E)
Figure 205: Middle East & Africa Market Value Share, by Country, 2023 (E)
Figure 206: Middle East & Africa Market Size (US$ Million) Analysis, 2012 to 2022
Figure 207: Middle East & Africa Market Size (US$ Million) & Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 208: Middle East & Africa Market Attractiveness Analysis, by Country
Figure 209: Middle East & Africa Market Attractiveness Analysis, By Diagnosis
Figure 210: Middle East & Africa Market Attractiveness Analysis, by Indication
Figure 211: Middle East & Africa Market Attractiveness Analysis, By End User
Figure 212: GCC Countries Market Value Proportion Analysis, 2022
Figure 213: Global Vs. GCC Countries Growth Comparison, 2022 to 2033
Figure 214: GCC Countries Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 215: GCC Countries Market Share Analysis (%), By Indication, 2022 & 2033
Figure 216: GCC Countries Market Share Analysis (%), By End User, 2022 & 2033
Figure 217: Israel Market Value Proportion Analysis, 2022
Figure 218: Global Vs. Israel Growth Comparison, 2022 to 2033
Figure 219: Israel Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 220: Israel Market Share Analysis (%), By Indication, 2022 & 2033
Figure 221: Israel Market Share Analysis (%), By End User, 2022 & 2033
Figure 222: Türkiye Market Value Proportion Analysis, 2022
Figure 223: Global Vs. Türkiye Growth Comparison, 2022 to 2033
Figure 224: Türkiye Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 225: Türkiye Market Share Analysis (%), By Indication, 2022 & 2033
Figure 226: Türkiye Market Share Analysis (%), By End User, 2022 & 2033
Figure 227: South Africa Market Value Proportion Analysis, 2022
Figure 228: Global Vs. South Africa Growth Comparison, 2022 to 2033
Figure 229: South Africa Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 230: South Africa Market Share Analysis (%), By Indication, 2022 & 2033
Figure 231: South Africa Market Share Analysis (%), By End User, 2022 & 2033
Figure 232: North Africa Market Value Proportion Analysis, 2022
Figure 233: Global Vs. North Africa Growth Comparison, 2022 to 2033
Figure 234: North Africa Market Share Analysis (%), By Diagnosis, 2022 & 2033
Figure 235: North Africa Market Share Analysis (%), By Indication, 2022 & 2033
Figure 236: North Africa Market Share Analysis (%), By End User, 2022 & 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Cognitive Supply Chain Market Forecast Outlook 2025 to 2035
Cognitive Computing Market Size and Share Forecast Outlook 2025 to 2035
Cognitive Impairment Biomarkers Market Size and Share Forecast Outlook 2025 to 2035
Cognitive Electronic Warfare Market Size and Share Forecast Outlook 2025 to 2035
Cognitive Agents Market Size and Share Forecast Outlook 2025 to 2035
Cognitive Network Market Size and Share Forecast Outlook 2025 to 2035
Cognitive Analytics Market Size and Share Forecast Outlook 2025 to 2035
Cognitive health supplements market analysis by product type, form, sales channel, functionality, and by region – Growth, trends, and Forecast from 2025 to 2035
Cognitive Neuroscience Market Overview – Trends & Research Insights 2024-2034
Cognitive Assessment and Training Market Report – Trends & Growth Forecast 2024-2034
Cognitive Data Management Market
Cognitive Security Market
Cognitive Systems Spending Market Report – Growth & Forecast 2016-2026
Content Analytics Discovery And Cognitive Systems Market Size and Share Forecast Outlook 2025 to 2035
Content Analytics, Discovery, and Cognitive Software Market Analysis by Product Type, End User, and Region through 2035
HIV Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
DNA Diagnostics Market Growth - Trends & Forecast 2024 to 2034
Food Diagnostics Services Market Size, Growth, and Forecast for 2025–2035
Rabies Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Cancer Diagnostics Market Analysis - Size, Share and Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA