The global antibiotic resistance diagnosis devices market is valued at USD 361.1 million in 2025. It is slated to reach USD 599.5 million by 2035, recording an absolute increase of USD 238.4 million over the forecast period. This translates into a total growth of 66%, with the market forecast to expand at a compound annual growth rate (CAGR) of 5.2% between 2025 and 2035. The overall market size is expected to grow by nearly 1.66X during the same period, supported by increasing prevalence of drug-resistant bacterial infections globally, growing adoption of rapid diagnostic technologies in clinical settings, and rising emphasis on antimicrobial stewardship programs that require accurate and timely identification of resistant pathogens across diverse hospital, laboratory, and point-of-care testing applications.
Between 2025 and 2030, the antibiotic resistance diagnosis devices market is projected to expand from USD 361.1 million to USD 465.3 million, resulting in a value increase of USD 104.2 million, which represents 43.7% of the total forecast growth for the decade. This phase of development will be shaped by increasing awareness of antimicrobial resistance as a global health threat, rising implementation of infection control protocols in healthcare facilities, and growing demand for automated diagnostic platforms that reduce turnaround times for susceptibility testing. Healthcare providers and clinical laboratories are expanding their diagnostic capabilities to address the growing need for rapid and accurate detection systems that enable appropriate antibiotic selection and support patient outcomes.
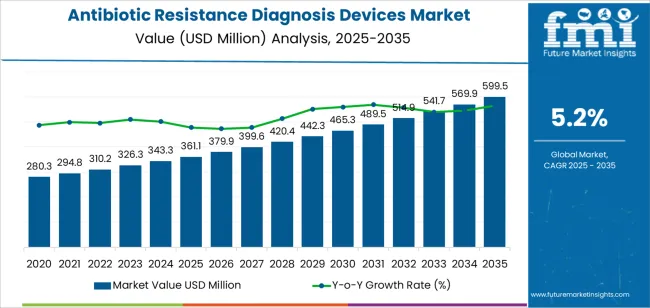
From 2030 to 2035, the market is forecast to grow from USD 465.3 million to USD 599.5 million, adding another USD 134.2 million, which constitutes 56.3% of the overall ten-year expansion. This period is expected to be characterized by the expansion of molecular diagnostic technologies for resistance gene detection, the development of integrated diagnostic platforms combining multiple detection methods, and the growth of decentralized testing capabilities in outpatient settings and community healthcare facilities. The growing adoption of digital health technologies and laboratory automation systems will drive demand for antibiotic resistance diagnosis devices with enhanced analytical performance and connectivity features.
Between 2020 and 2025, the antibiotic resistance diagnosis devices market experienced steady growth, driven by increasing recognition of antimicrobial resistance as a critical public health challenge and growing implementation of diagnostic technologies that enable targeted antibiotic therapy. The market developed as infectious disease specialists and laboratory directors recognized the potential for advanced diagnostic devices to reduce inappropriate antibiotic use, improve treatment outcomes, and contain the spread of resistant organisms through rapid identification and susceptibility testing. Technological advancement in molecular diagnostics and automated susceptibility testing began emphasizing the critical importance of maintaining diagnostic accuracy and operational efficiency in clinical laboratory environments.
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 361.1 million |
| Forecast Value in (2035F) | USD 599.5 million |
| Forecast CAGR (2025 to 2035) | 5.2% |
Market expansion is being supported by the increasing global burden of antibiotic-resistant infections and healthcare-associated infections driven by overuse of antimicrobial agents and inadequate infection control practices, alongside the corresponding need for advanced diagnostic devices that can rapidly identify resistant pathogens, enable targeted antibiotic therapy, and support antimicrobial stewardship initiatives across various hospital laboratories, reference laboratories, and point-of-care testing environments. Modern healthcare providers and laboratory professionals are increasingly focused on implementing antibiotic resistance diagnosis devices that can reduce diagnostic turnaround times, improve treatment decision-making, and provide accurate susceptibility profiles in clinical practice settings.
The growing emphasis on antimicrobial stewardship and infection prevention programs is driving demand for diagnostic devices that can support evidence-based antibiotic selection, enable real-time surveillance of resistance patterns, and ensure comprehensive monitoring of treatment effectiveness. Healthcare organizations' preference for diagnostic technologies that combine rapid results with clinical reliability and operational efficiency is creating opportunities for innovative device implementations. The rising influence of regulatory requirements for resistance monitoring and public health surveillance initiatives is also contributing to increased adoption of diagnostic devices that can provide standardized susceptibility data without compromising diagnostic accuracy or laboratory workflow efficiency.
The market is segmented by diagnostic method, application, and region. By diagnostic method, the market is divided into AST devices, nucleic acid detection devices, and mass spectrometry rapid identification devices. Based on application, the market is categorized into hospital, third-party testing agency, and other. Regionally, the market is divided into East Asia, Europe, North America, South Asia, Latin America, Middle East & Africa, and Eastern Europe.
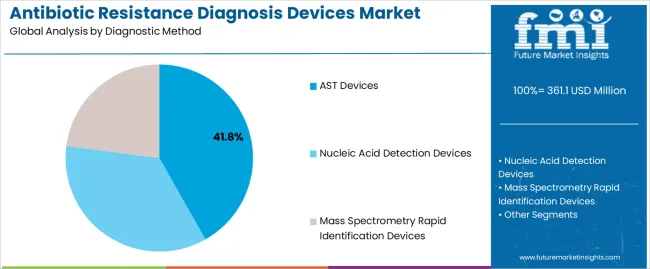
The AST devices segment is projected to maintain its leading position in the antibiotic resistance diagnosis devices market in 2025 with a 41.8% market share, reaffirming its role as the preferred diagnostic technology for antimicrobial susceptibility testing and resistance pattern identification. Clinical laboratories and hospital microbiology departments increasingly utilize AST devices for their comprehensive antibiotic panel coverage, standardized testing protocols, and proven effectiveness in guiding therapeutic decisions while providing quantitative susceptibility data. AST device technology's established clinical validation and regulatory acceptance directly address the healthcare requirements for reliable susceptibility testing that supports antimicrobial stewardship programs and infection management strategies across diverse clinical settings and patient populations.
This diagnostic method segment forms the foundation of routine resistance testing, as it represents the technology with the greatest contribution to clinical decision support and established performance standards across multiple bacterial species and antibiotic classes. Healthcare system investments in laboratory automation and quality assurance continue to strengthen adoption among hospital laboratories and reference testing facilities. With increasing pressure to optimize antibiotic prescribing and control resistance emergence, AST devices align with both clinical objectives and stewardship requirements, making them the central component of comprehensive resistance monitoring strategies.
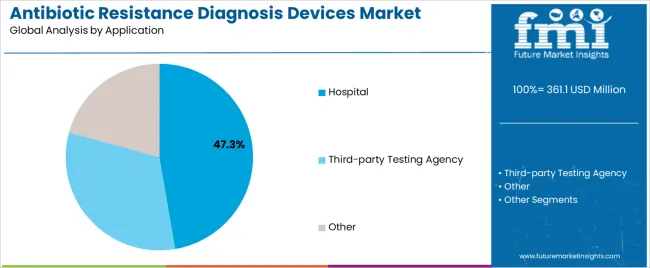
The hospital application segment is projected to represent the largest share of antibiotic resistance diagnosis devices demand in 2025 with a 47.3% market share, underscoring its critical role as the primary setting for resistance testing across emergency departments, intensive care units, and inpatient wards requiring rapid diagnostic results. Hospital laboratories prefer antibiotic resistance diagnosis devices due to their integration capabilities with laboratory information systems, ability to process high sample volumes, and capacity to deliver timely results that influence treatment decisions while supporting infection control protocols. Positioned as essential technologies for modern hospital microbiology services, these diagnostic devices offer both clinical utility and operational advantages.
The segment is supported by continuous advancement in automated testing platforms and the growing availability of rapid diagnostic methods that enable same-day susceptibility results with minimal hands-on time and enhanced workflow efficiency. Additionally, hospitals are investing in comprehensive diagnostic programs to support antimicrobial stewardship teams, infection prevention initiatives, and quality improvement efforts focused on reducing inappropriate antibiotic use. As healthcare-associated infections increase and resistance patterns evolve, the hospital application will continue to dominate the market while supporting expanded diagnostic capabilities and patient safety initiatives.
The antibiotic resistance diagnosis devices market is advancing steadily due to increasing prevalence of multidrug-resistant organisms driven by antibiotic overuse in healthcare and agricultural settings and growing implementation of antimicrobial stewardship programs that require diagnostic technologies providing rapid and accurate resistance detection capabilities across diverse hospital, outpatient, and reference laboratory applications. The market faces challenges, including high capital costs for automated diagnostic platforms, reimbursement limitations for advanced testing methods, and technical constraints related to standardization of testing protocols and interpretation criteria across different bacterial species and resistance mechanisms. Innovation in molecular diagnostic technologies and artificial intelligence applications continues to influence product development and market expansion patterns.
The growing adoption of molecular diagnostic methods is driving demand for nucleic acid-based devices that can rapidly detect specific resistance genes and mechanisms without requiring bacterial culture, enabling treatment decisions within hours rather than days. Molecular diagnostic applications require devices that deliver high sensitivity and specificity for resistance marker detection while maintaining cost-effectiveness and ease of use in routine laboratory workflows. Healthcare providers are increasingly recognizing the clinical advantages of rapid molecular testing for critical care situations and outbreak investigations, creating opportunities for innovative diagnostic platforms designed for specific resistance targets and syndromic testing approaches.
Modern diagnostic device manufacturers are incorporating laboratory automation, digital connectivity, and data analytics capabilities to enhance testing efficiency, reduce manual intervention, and support comprehensive resistance surveillance through standardized data collection and reporting. Leading companies are developing integrated diagnostic platforms with automated specimen processing, implementing connectivity solutions for real-time data sharing with electronic health records, and advancing analytical algorithms that improve result interpretation and clinical decision support. These developments improve operational capabilities while enabling new applications, including population health monitoring, resistance trend analysis, and antimicrobial stewardship program optimization. Advanced technology integration also allows healthcare organizations to support quality assurance objectives and regulatory compliance beyond traditional diagnostic testing functions.
The expansion of outpatient care, urgent care centers, and community healthcare facilities is driving demand for point-of-care diagnostic devices that enable resistance testing outside traditional laboratory settings, providing rapid results that facilitate appropriate antibiotic prescribing at the point of patient encounter. These applications require compact diagnostic platforms with simplified workflows and minimal technical expertise requirements, creating market segments with differentiated product specifications and service models. Manufacturers are investing in miniaturized testing technologies and sample preparation systems to enable decentralized testing while maintaining diagnostic accuracy and regulatory compliance standards.
| Country | CAGR (2025-2035) |
|---|---|
| China | 7% |
| India | 6.5% |
| Germany | 6% |
| Brazil | 5.5% |
| United States | 4.9% |
| United Kingdom | 4.4% |
| Japan | 3.9% |
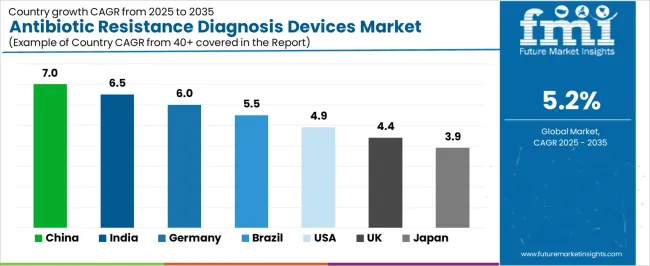
The antibiotic resistance diagnosis devices market is experiencing solid growth globally, with China leading at a 7% CAGR through 2035, driven by expanding healthcare infrastructure, increasing hospital-acquired infection rates, and growing government initiatives for antimicrobial resistance surveillance and control programs. India follows at 6.5%, supported by rising awareness of resistance challenges, expanding private hospital networks, and increasing investment in diagnostic laboratory capabilities. Germany shows growth at 6%, emphasizing advanced laboratory diagnostics, strict infection control standards, and comprehensive antimicrobial stewardship implementation.
Brazil demonstrates 5.5% growth, supported by healthcare system modernization, increasing hospital capacity, and expanding access to diagnostic technologies. The United States records 4.9%, focusing on regulatory compliance requirements, antimicrobial stewardship mandates, and advanced molecular diagnostic adoption. The United Kingdom exhibits 4.4% growth, emphasizing public health surveillance systems, laboratory consolidation initiatives, and resistance monitoring programs. Japan shows 3.9% growth, supported by aging population healthcare needs, quality-focused diagnostic practices, and infection prevention priorities.
The report covers an in-depth analysis of 40+ countries, the top-performing countries are highlighted below.
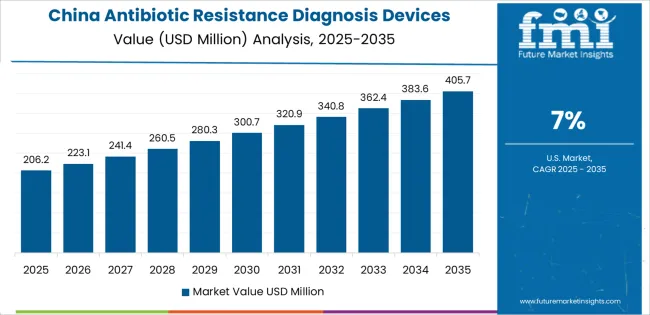
Revenue from antibiotic resistance diagnosis devices in China is projected to exhibit exceptional growth with a CAGR of 7% through 2035, driven by expanding hospital infrastructure and rapidly growing awareness of antimicrobial resistance challenges supported by government Healthy China 2030 initiatives and national action plans for resistance containment. The country's massive healthcare system expansion and increasing investment in laboratory diagnostic capabilities are creating substantial demand for antibiotic resistance diagnosis device solutions. Major hospital groups and diagnostic companies are establishing comprehensive resistance testing programs to serve both tertiary care facilities and regional healthcare networks.
Revenue from antibiotic resistance diagnosis devices in India is expanding at a CAGR of 6.5%, supported by the country's growing burden of antibiotic-resistant infections, expanding private healthcare sector, and increasing implementation of antimicrobial stewardship programs in tertiary care hospitals and diagnostic chains. The country's comprehensive healthcare development initiatives and rising awareness of resistance challenges are driving demand for advanced diagnostic capabilities throughout urban healthcare systems. Leading hospital networks and international diagnostic companies are establishing testing facilities and distribution networks to address growing demand for resistance detection technologies.
Revenue from antibiotic resistance diagnosis devices in Germany is expanding at a CAGR of 6%, supported by the country's advanced laboratory diagnostic infrastructure, stringent infection prevention regulations, and comprehensive antimicrobial stewardship implementation across hospital and outpatient care settings. The nation's healthcare quality emphasis and regulatory framework are driving sophisticated diagnostic capabilities throughout clinical microbiology services. Leading hospital systems and laboratory service providers are investing extensively in automated diagnostic platforms and molecular testing technologies.
Revenue from antibiotic resistance diagnosis devices in Brazil is expanding at a CAGR of 5.5%, supported by the country's healthcare system modernization efforts, increasing hospital network expansion, and growing recognition of antimicrobial resistance as a significant public health challenge. Brazil's developing diagnostic infrastructure and healthcare investment are driving demand for resistance testing solutions. Hospital chains and diagnostic laboratory networks are investing in testing capabilities to serve public and private healthcare sectors.
Revenue from antibiotic resistance diagnosis devices in the United States is expanding at a CAGR of 4.9%, supported by the country's regulatory requirements for antimicrobial stewardship programs, established infection prevention infrastructure, and advanced molecular diagnostic capabilities for resistance detection. The nation's comprehensive healthcare quality initiatives and reimbursement policies are driving demand for diagnostic device solutions. Hospital systems and reference laboratories are investing in testing capacity expansion and technology upgrades to meet regulatory requirements and clinical needs.
Revenue from antibiotic resistance diagnosis devices in the United Kingdom is expanding at a CAGR of 4.4%, driven by the country's national antimicrobial resistance surveillance programs, laboratory service consolidation initiatives, and integrated public health monitoring systems supporting resistance tracking and intervention strategies. The United Kingdom's National Health Service structure and public health priorities are driving demand for standardized diagnostic capabilities. Hospital trusts and consolidated laboratory services are investing in diagnostic technologies for resistance monitoring and stewardship support.
Revenue from antibiotic resistance diagnosis devices in Japan is expanding at a CAGR of 3.9%, supported by the country's aging population healthcare requirements, emphasis on infection prevention in long-term care settings, and established quality standards for clinical laboratory testing. Japan's healthcare system maturity and diagnostic precision focus are driving demand for reliable resistance testing products. Hospital laboratories and diagnostic service providers are investing in automated testing capabilities for routine susceptibility determination.
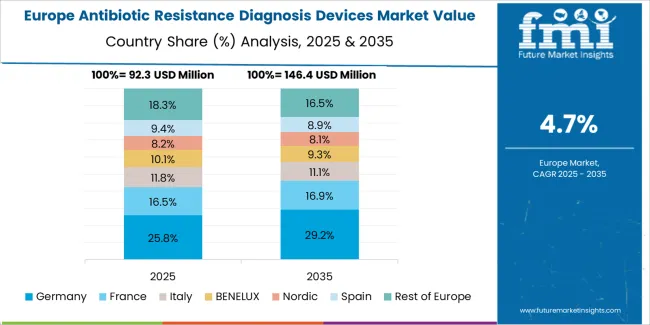
The antibiotic resistance diagnosis devices market in Europe is projected to grow from USD 134.5 million in 2025 to USD 217 million by 2035, registering a CAGR of 4.9% over the forecast period. Germany is expected to maintain leadership with a 26.3% market share in 2025, moderating to 25.8% by 2035, supported by advanced laboratory infrastructure, stringent antimicrobial stewardship regulations, and comprehensive infection surveillance systems.
The United Kingdom follows with 19.2% in 2025, projected at 18.9% by 2035, driven by national resistance monitoring programs, NHS laboratory consolidation, and public health surveillance priorities. France holds 16.7% in 2025, rising to 16.9% by 2035 on the back of hospital infection control initiatives and diagnostic laboratory modernization. Italy commands 12.4% in 2025, increasing slightly to 12.6% by 2035, while Spain accounts for 8.9% in 2025, reaching 9.1% by 2035 aided by healthcare system improvements and hospital quality programs.
The Netherlands maintains 5.3% in 2025, up to 5.4% by 2035 due to integrated healthcare networks and antimicrobial stewardship excellence. The Rest of Europe region, including Nordics, Central & Eastern Europe, and other markets, is anticipated to hold 11.2% in 2025 and 11.3% by 2035, reflecting steady progress in resistance surveillance implementation, laboratory diagnostic expansion, and infection prevention program development.
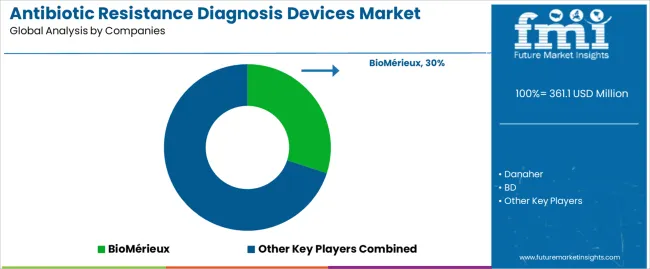
The antibiotic resistance diagnosis devices market is characterized by competition among established diagnostic companies, specialized microbiology technology providers, and diversified healthcare equipment manufacturers. Companies are investing in automated platform development, molecular diagnostic innovation, product portfolio expansion, and clinical validation studies to deliver accurate, rapid, and user-friendly diagnostic solutions. Innovation in rapid testing technologies, resistance mechanism detection, and laboratory automation integration is central to strengthening market position and competitive advantage.
BioMérieux leads the market with comprehensive microbiology diagnostic solutions with a focus on automated susceptibility testing systems, bloodstream infection diagnostics, and syndromic testing panels across diverse hospital and reference laboratory applications. Danaher provides innovative diagnostic technologies through its Beckman Coulter and Cepheid subsidiaries with emphasis on automated microbiology systems and molecular diagnostic platforms. BD delivers advanced diagnostic solutions with focus on automated blood culture systems and susceptibility testing instruments. Thermo Fisher Scientific offers comprehensive laboratory diagnostic products with emphasis on microbiology testing and molecular detection technologies. Deere provides specialized diagnostic equipment with focus on clinical laboratory applications.
Meihua specializes in diagnostic device manufacturing for Asian markets with emphasis on susceptibility testing systems. Xinke focuses on clinical diagnostic equipment production with growing presence in hospital microbiology. Roche provides molecular diagnostic platforms and laboratory automation solutions for resistance detection and infectious disease testing applications.
Antibiotic resistance diagnosis devices represent a critical healthcare technology segment within clinical microbiology and infectious disease management, projected to grow from USD 361.1 million in 2025 to USD 599.5 million by 2035 at a 5.2% CAGR. These specialized diagnostic systems serve as essential tools for susceptibility testing, resistance mechanism detection, and antimicrobial stewardship support where rapid pathogen identification, accurate susceptibility determination, and clinical decision support are critical. Market expansion is driven by increasing prevalence of drug-resistant infections, growing implementation of antimicrobial stewardship mandates, expanding molecular diagnostic adoption, and rising emphasis on infection prevention protocols across diverse hospital, reference laboratory, and public health surveillance applications.
How Healthcare Regulators Could Strengthen Diagnostic Standards and Clinical Utility?
How Healthcare Associations Could Advance Testing Standards and Stewardship Implementation?
How Device Manufacturers Could Drive Innovation and Clinical Impact?
How Healthcare Facilities Could Optimize Diagnostic Capabilities and Patient Care?
How Research Institutions Could Enable Technology Advancement?
How Healthcare Payers Could Support Diagnostic Access and Stewardship?
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 361.1 million |
| Diagnostic Method | AST Devices, Nucleic Acid Detection Devices, Mass Spectrometry Rapid Identification Devices |
| Application | Hospital, Third-party Testing Agency, Other |
| Regions Covered | East Asia, Europe, North America, South Asia, Latin America, Middle East & Africa, Eastern Europe |
| Countries Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | BioMérieux, Danaher, BD, Thermo Fisher Scientific, Deere |
| Additional Attributes | Dollar sales by diagnostic method and application category, regional demand trends, competitive landscape, technological advancements in resistance detection, antimicrobial stewardship integration, regulatory compliance developments, and clinical validation progress |
The global antibiotic resistance diagnosis devices market is estimated to be valued at USD 361.1 million in 2025.
The market size for the antibiotic resistance diagnosis devices market is projected to reach USD 599.5 million by 2035.
The antibiotic resistance diagnosis devices market is expected to grow at a 5.2% CAGR between 2025 and 2035.
The key product types in antibiotic resistance diagnosis devices market are ast devices, nucleic acid detection devices and mass spectrometry rapid identification devices.
In terms of application, hospital segment to command 47.3% share in the antibiotic resistance diagnosis devices market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Antibiotics Active Pharmaceutical Ingredient (API) Market - Growth & Forecast 2025 to 2035
Global Antibiotic-Resistant Infections Treatment Market Analysis – Size, Share & Forecast 2024-2034
Global Antibiotic Drug Market Analysis – Size, Share & Forecast 2023-2033
Antibiotic Susceptibility Testing Devices Market Size and Share Forecast Outlook 2025 to 2035
Antibiotic Resistance Testing and Diagnostic Devices Market Size and Share Forecast Outlook 2025 to 2035
Animal Antibiotics and Antimicrobials Market Size and Share Forecast Outlook 2025 to 2035
Topical Antibiotic Pharmaceuticals Market Size and Share Forecast Outlook 2025 to 2035
Peptide Antibiotics Market
Veterinary Antibiotics Market Size and Share Forecast Outlook 2025 to 2035
Animal Feed Antibiotics Market - Size, Share, and Forecast Outlook 2025 to 2035
Intracameral Antibiotics Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Probiotics After Antibiotic Recovery Market Analysis by Ingredient and Sales Channel Through 2035
Animal Antimicrobials and Antibiotics Market Size and Share Forecast Outlook 2025 to 2035
Resistance Bands Market Outlook – Size, Share & Forecast 2025 to 2035
Ground Resistance Testers Market Growth - Trends & Forecast 2025 to 2035
Battery Resistance Tester Market Size and Share Forecast Outlook 2025 to 2035
Electric Resistance Welded (ERW) Pipes and Tubes Market Analysis by Type, Application and Region: Forecast for 2025 to 2035
Chemical Resistance Film Market Trends & Forecast 2024-2034
Polymyxin Resistance Testing Market Trends – Innovations & Growth 2025 to 2035
Low Rolling Resistance Tire Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA