The Cystatin C Testing Market is estimated to be valued at USD 259.5 million in 2025 and is projected to reach USD 534.8 million by 2035, registering a compound annual growth rate (CAGR) of 7.5% over the forecast period.
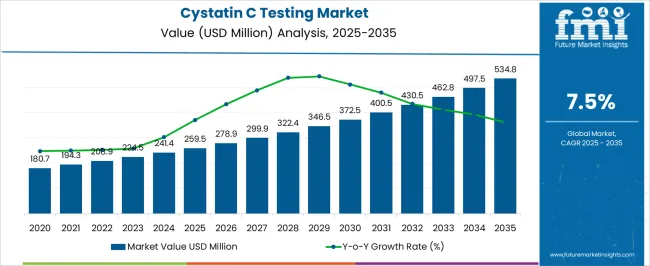
| Metric | Value |
|---|---|
| Cystatin C Testing Market Estimated Value in (2025 E) | USD 259.5 million |
| Cystatin C Testing Market Forecast Value in (2035 F) | USD 534.8 million |
| Forecast CAGR (2025 to 2035) | 7.5% |
The Cystatin C testing market is experiencing accelerated growth due to increasing awareness around chronic kidney disease (CKD), cardiovascular risk assessment, and the limitations of traditional creatinine-based testing. Cystatin C is emerging as a more reliable endogenous biomarker for estimating glomerular filtration rate (GFR), especially in populations where creatinine-based testing may be compromised due to age, gender, or muscle mass variations.
A notable shift has been observed among healthcare providers and clinical laboratories toward adopting Cystatin C assays for early and precise detection of renal dysfunction. This is being supported by continuous advancements in immunoassay technologies and their integration into automated diagnostic platforms.
Additionally, the increasing inclusion of Cystatin C measurements in clinical guidelines for CKD and cardiovascular risk stratification is paving the way for higher adoption Over the coming years, market growth is expected to be sustained by ongoing product innovations, point-of-care testing capabilities, and increased testing volumes in both developed and emerging healthcare markets driven by an aging global population and expanding disease surveillance efforts.
The market is segmented by Product, Test Type, Application, and End User and region. By Product, the market is divided into Kits, Instruments, and Reagents. In terms of Test Type, the market is classified into ELISA Based Tests, Colorimetric Assay Based Tests, Enzymatic Tests, Point of Care Tests, and Others. Based on Application, the market is segmented into Diagnosis Use and Research and Development. By End User, the market is divided into Hospitals, Clinics, Diagnostic Laboratories, Renal Care Centers, and Academic Research Institutes. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
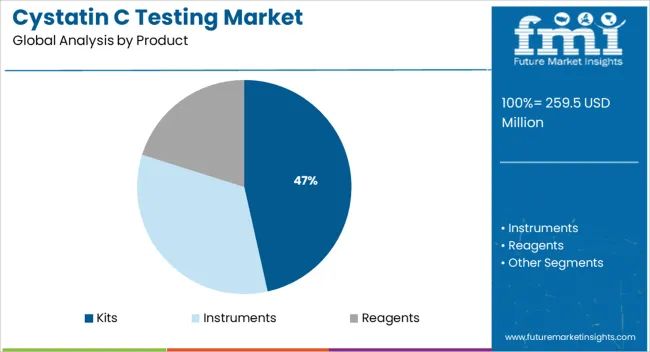
Kits are projected to account for 46.5% of the total revenue share in the Cystatin C testing market in 2025, establishing them as the leading product category. The dominance of this segment is attributed to the widespread use of diagnostic kits in clinical laboratories for their ease of use, standardization, and compatibility with various analyzers.
The demand for ready-to-use reagent kits has risen significantly as healthcare systems increasingly prioritize faster turnaround times, cost-efficiency, and automation-ready solutions. Manufacturers have focused on enhancing kit stability, shelf life, and performance consistency, which has further strengthened their adoption across high-throughput laboratory settings.
Regulatory approvals and inclusion in quality assurance programs have also played a crucial role in improving clinician confidence and supporting broader clinical use The ability of these kits to deliver high specificity and sensitivity in Cystatin C detection underpins their role in routine testing protocols, particularly for early detection and monitoring of kidney function in patients with complex medical profiles.
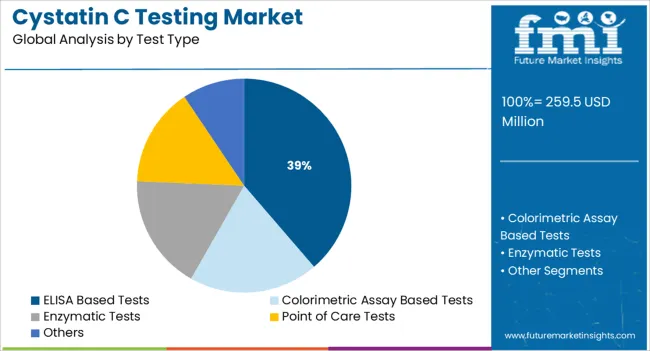
ELISA based tests are expected to contribute 38.7% of the total revenue share in the Cystatin C testing market by 2025, making them the leading test type segment. Their growth is being supported by the widespread use of ELISA platforms in research and diagnostic laboratories due to their analytical accuracy and adaptability to various clinical applications.
The format's high specificity for antigen-antibody reactions has enabled consistent detection of Cystatin C levels even at low concentrations, making it a preferred choice for clinical diagnostics. The integration of ELISA kits into automated systems has improved laboratory efficiency and reduced manual errors, further encouraging adoption.
Innovations in microplate design, signal detection technologies, and assay sensitivity have contributed to enhanced performance, especially in early-stage renal dysfunction identification The ongoing demand for cost-effective and scalable testing formats, especially in decentralized laboratory settings, continues to favor the ELISA segment’s expansion in both high and middle-income countries.
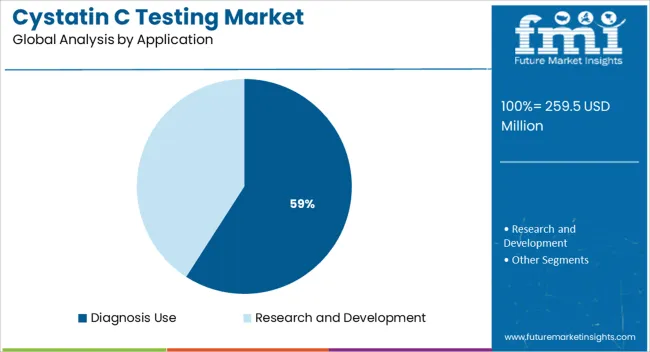
Diagnosis use is anticipated to capture 59.1% of the total revenue share in the Cystatin C testing market in 2025, marking it as the dominant application segment. This growth is being driven by the clinical necessity for reliable early-stage kidney function assessments and risk stratification in chronic disease management.
Cystatin C testing is increasingly being integrated into diagnostic algorithms for renal impairment, cardiovascular health evaluation, and in certain cases, sepsis prognosis. As healthcare providers aim to improve patient outcomes through early detection and personalized treatment strategies, the reliance on Cystatin C as a superior biomarker has become more prominent.
Hospital laboratories, nephrology clinics, and cardiology departments are actively utilizing these tests to supplement or replace creatinine-based estimations, particularly in patients with fluctuating renal profiles The increased inclusion of Cystatin C in medical guidelines and decision-support tools has further reinforced its diagnostic relevance, positioning it as an essential component of modern clinical practice in both acute and chronic care scenarios.
The increasing prevalence of several disorders such as myocardial infarction, and muscular dystrophy is one of the major factors that is accelerating the market growth. Besides this, the favorable reimbursement policies in the healthcare sector are a great advantage for cystatin C testing.
Furthermore, the growth in the number of pathology laboratories, hospital laboratories, research labs, and diagnostic centers across the geographies is boosting the market growth for the cystatin C testing market. Apart from this, the low cost of the reagents is also one of the major market stimulating factors. The increased investments in the healthcare sector by the government organizations of several nations are also expected to favor the cystatin C testing market.
Moreover, the major businesses in the market are highly investing in research and development activities, to offer novel and high-quality testing kits and products to the end users such as diagnostic laboratories, clinics, and hospitals. Besides this, the key market players are concentrating on activities like mergers and acquisitions. Due to these activities, the market is expected to grow at a rapid pace in the near future.
North America held the largest share in the global cystatin C testing market and is expected to continue in the near future. The growing healthcare sector and the launch of novel products in the region are expected to accelerate the market during the forecast period. Europe is also estimated to witness lucrative growth over the projected period, owing to the advanced and developed healthcare facilities.
The increasing disposable income along with the rising health awareness, and increasing demand for diagnostic procedures especially in the geriatric population is the major factor that is expected to fuel the market growth in Asia Pacific Region.
Some of the key manufacturers operating in the cystatin C testing market are Abbott, Eurolyser Diagnostica, Randox Laboratories, DiaSys Diagnostic Systems, Diazyme Laboratories, Tosoh India Pvt. Ltd., BBI Solutions, PerkinElmer, Siemens Healthcare Private Limited, and Pointe Scientific Inc.
| Report Attributes | Details |
|---|---|
| Growth Rate | CAGR of 7.5% 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2012 to 2024 |
| Forecast Period | 2025 to 2035 |
| Qualitative Units | Revenue in USD Billion and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segment Covered | Product, Test, Application, End User, Region |
| Region Covered | North America; Latin America; Europe; East Asia; South Asia; Oceania; Middle East Africa |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, UK, France,Spain, Italy, China, Japan, South Korea, Malaysia, Singapore, Australia, New Zealand, GCC, South Africa, Israel |
| Key Players | Abbott; Eurolyser Diagnostica; Randox Laboratories; DiaSys Diagnostic Systems; Diazyme Laboratories; Tosoh India Pvt. Ltd.; BBI Solutions; PerkinElmer; Siemens Healthcare Private Limited; Pointe Scientific Inc. |
| Customization | Available Upon Request |
The global cystatin C testing market is estimated to be valued at USD 259.5 million in 2025.
The market size for the cystatin C testing market is projected to reach USD 534.8 million by 2035.
The cystatin C testing market is expected to grow at a 7.5% CAGR between 2025 and 2035.
The key product types in cystatin C testing market are kits, instruments and reagents.
In terms of test type, elisa based tests segment to command 38.7% share in the cystatin C testing market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Comprehensive Management Solution for Power Quality Market Size and Share Forecast Outlook 2025 to 2035
Conveyor Belt Tracking Devices Market Size and Share Forecast Outlook 2025 to 2035
Cryogenic Helium Cycling System Market Size and Share Forecast Outlook 2025 to 2035
Copper Chrome Black Market Size and Share Forecast Outlook 2025 to 2035
Compostable Pouch Market Size and Share Forecast Outlook 2025 to 2035
Cloud Service Market Size and Share Forecast Outlook 2025 to 2035
Cybersecurity Insurance Market Size and Share Forecast Outlook 2025 to 2035
Cupcake Box Market Size and Share Forecast Outlook 2025 to 2035
Custom Packaging Box Market Size and Share Forecast Outlook 2025 to 2035
Clay Coated Recycled Boxboard Market Size and Share Forecast Outlook 2025 to 2035
Corrugated Box Machine Market Size and Share Forecast Outlook 2025 to 2035
Camera Accessories Market Size and Share Forecast Outlook 2025 to 2035
Compact Pneumatic Pressure Sensor Market Size and Share Forecast Outlook 2025 to 2035
Cobalt Salt for Tires Market Size and Share Forecast Outlook 2025 to 2035
Cobalt Based Laser Cladding Powder Market Size and Share Forecast Outlook 2025 to 2035
Cavity Drainage Membrane Market Size and Share Forecast Outlook 2025 to 2035
Can Stack Motors Market Size and Share Forecast Outlook 2025 to 2035
Carboxymethyl Tamarind Kernel Powder Market Size and Share Forecast Outlook 2025 to 2035
Cyclic Polyolefin Market Size and Share Forecast Outlook 2025 to 2035
Clay Coated Paper Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA