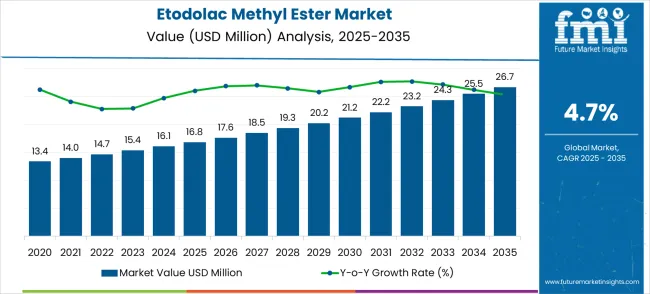
The etodolac methyl ester market is set to grow from USD 16.8 million in 2025 to USD 26.7 million by 2035, advancing at a 4.7% CAGR. The growth is driven by the rising demand for high-purity pharmaceutical intermediates and the increasing adoption of advanced synthetic methodologies in drug manufacturing. The pharmaceutical intermediates segment will dominate with 78.0% of the market share, supported by the demand for high-purity compounds for anti-inflammatory and pain management drugs. Purity ≥99% will be the leading purity level, accounting for 65% of the market share, as it meets stringent pharmaceutical manufacturing standards.
As populations age and lifestyle-related diseases rise, the demand for effective pain management therapies continues to grow. Etodolac, as an NSAID, is frequently prescribed for conditions such as osteoarthritis, rheumatoid arthritis, and musculoskeletal pain. The methyl ester form of etodolac is preferred in certain formulations due to its improved solubility, making it easier to deliver effective doses in controlled-release systems. This growing demand for pain relief and anti-inflammatory drugs is expected to drive the market for etodolac methyl ester as an essential component in pharmaceutical formulations.
Research and development activities in the pharmaceutical and chemical industries are also contributing to market growth. Etodolac methyl ester is used in various research applications to develop new formulations, improve drug efficacy, and explore novel therapeutic options. The growing focus on the development of new NSAIDs and combination therapies further expands the market potential for etodolac methyl ester. Additionally, as research into the broader applications of methyl esters continues to grow, the demand for etodolac methyl ester in pharmaceutical research is likely to increase, further supporting market expansion.
Technological advancements in the synthesis of etodolac methyl ester are improving production efficiency and reducing costs, which is expected to boost market growth. Advances in manufacturing processes and scaling techniques are making it easier to produce high-quality etodolac methyl ester at competitive prices, thereby making it more accessible to pharmaceutical companies. These innovations are expected to improve the overall profitability of the market and attract more players into the industry.
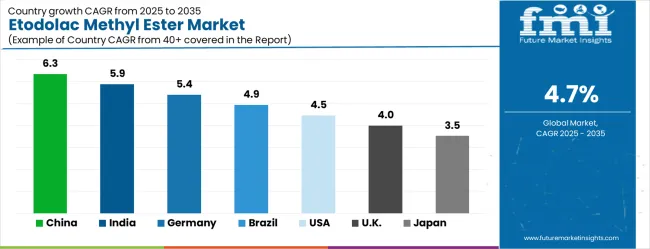
The Asia-Pacific region leads growth, with China (6.3% CAGR) and India (5.9% CAGR) driven by the rapid expansion of pharmaceutical infrastructure and increasing demand for high-purity intermediates. Germany (5.4% CAGR) follows, benefiting from its established pharmaceutical manufacturing infrastructure and strong focus on synthesis quality. The United States (4.5% CAGR) and the United Kingdom (4.0% CAGR) are expected to show steady growth, driven by regulatory compliance and pharmaceutical manufacturing innovation. Competitive advantage in this market lies in advanced purification technologies, quality assurance systems, and specialized manufacturing capabilities, with key players such as Anhui Jinquan Biotechnology, NINGBO INNO PHARMCHEM, and Watson International Limited leading the market.
| Etodolac Methyl Ester Market | Value |
|---|---|
| Market Value (2025) | USD 16.8 million |
| Market Forecast Value (2035) | USD 26.7 million |
| Market Forecast CAGR | 4.7% |
The etodolac methyl ester market has been evaluated as a niche but influential segment across pharmaceutical and chemical industries, accounting for nearly 6.9% of the pharmaceutical intermediates market, 5.4% of the active pharmaceutical ingredients (API) market, 7.6% of the anti-inflammatory drugs market, 6.1% of the pain management drugs market, and 3.8% of the specialty chemicals market. Together, this contributes to a combined market share of around 29.8% across its parent domains. This indicates how etodolac methyl ester is being valued for its role in enhancing drug formulation processes, improving therapeutic delivery, and supporting the production of effective anti-inflammatory treatments.
Market expansion is being supported by the rapid increase in pharmaceutical research and development activities worldwide and the corresponding need for high-purity intermediate compounds that provide superior performance and synthesis reliability. Modern pharmaceutical manufacturers rely on consistent intermediate quality and purity standards to ensure optimal drug development including anti-inflammatory formulations, pain management therapeutics, and specialized pharmaceutical applications. Even minor purity variations can require comprehensive synthesis protocol adjustments to maintain optimal quality standards and manufacturing performance.
The growing complexity of pharmaceutical synthesis requirements and increasing demand for high-purity intermediate solutions are driving demand for etodolac methyl ester from certified manufacturers with appropriate quality capabilities and technical expertise. Pharmaceutical companies are increasingly requiring documented purity standards and compound reliability to maintain synthesis quality and manufacturing effectiveness. Industry specifications and quality standards are establishing standardized pharmaceutical manufacturing procedures that require specialized intermediate compounds and trained synthesis operators.
The etodolac methyl ester market is entering a transformative growth phase driven by increasing pharmaceutical research initiatives, rising drug development complexity, and technological advancement. As pharmaceutical manufacturers across both developed and emerging markets seek intermediates that deliver superior purity, synthesis reliability, and comprehensive quality capabilities, etodolac methyl ester is evolving from basic intermediate compound to sophisticated pharmaceutical solution integrated with advanced synthesis protocols, quality assurance systems, and specialized purification technologies.
Rising pharmaceutical production and drug development activities in Asia-Pacific, Latin America, and Middle East & Africa amplify demand, while manufacturers are advancing innovations in synthesis optimization, purity enhancement, and quality control improvements. Pathways like green chemistry integration, specialized formulation capabilities, and sustainability-enabled systems promise strong margin uplift, especially in mature markets. Geographic expansion and localization will capture volume growth, particularly where pharmaceutical manufacturing infrastructure is developing or modernization programs are underway. Regulatory pressures around drug quality standards, synthesis accuracy requirements, and pharmaceutical manufacturing integration provide structural market support.
The market is segmented by purity level, application, and region. By purity level, the market is divided into Purity <99%, Purity ≥99%, and others. Based on application, the market is categorized into pharmaceutical intermediates, research applications, and others. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
In 2025, the purity ≥99% etodolac methyl ester segment is projected to capture around 65% of the total market share, making it the leading purity category. This dominance is largely driven by the widespread demand for high-purity pharmaceutical intermediates that deliver reliable synthesis performance with excellent quality across various pharmaceutical applications. The purity ≥99% segment is particularly favored for its ability to provide consistent synthesis results in both research and commercial manufacturing scenarios, ensuring operational flexibility for pharmaceutical companies. Drug development laboratories, pharmaceutical manufacturers, contract research organizations, and specialized synthesis facilities increasingly prefer this purity level, as it meets stringent pharmaceutical quality standards without imposing excessive purification costs or complex handling requirements. The availability of well-established purification processes, along with comprehensive quality documentation and technical support from leading suppliers, further reinforces the segment's market position. Additionally, this purity category benefits from consistent demand across regions, as it is considered the standard solution for companies with varying pharmaceutical synthesis requirements. The combination of proven quality, synthesis reliability, and regulatory compliance makes purity ≥99% etodolac methyl ester a preferred choice, ensuring continued popularity in the pharmaceutical intermediate market. Advanced purification capabilities and enhanced quality control features continue to drive adoption among professional pharmaceutical manufacturers seeking superior synthesis performance.
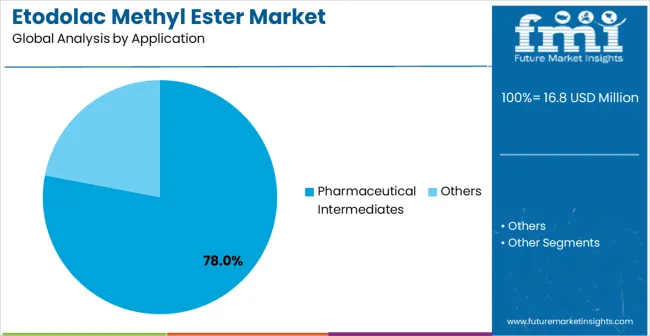
The pharmaceutical intermediates segment is expected to represent 78% of etodolac methyl ester demand in 2025, highlighting its position as the most significant application sector. This dominance stems from the unique requirements of pharmaceutical synthesis processes, where consistent quality and reliable intermediate performance are critical to effective drug development. Pharmaceutical intermediate applications often feature complex synthesis protocols that require continuous quality monitoring throughout extended manufacturing campaigns, demanding dependable and pure intermediate compounds. Etodolac methyl ester is particularly well-suited to these applications due to its ability to deliver consistent synthesis results efficiently and effectively, even during periods of intensive production schedules. As pharmaceutical companies expand globally and emphasize improved drug quality standards, the demand for high-purity etodolac methyl ester continues to rise. The segment also benefits from heightened focus on pharmaceutical innovation initiatives, where drug manufacturers are increasingly prioritizing reliable intermediate compounds and consistent synthesis performance as key components of comprehensive drug development programs. With pharmaceutical companies investing in manufacturing excellence and product quality enhancement, etodolac methyl ester provides an essential solution to maintain high-performance synthesis capabilities. The growth of organized pharmaceutical manufacturing programs, coupled with increased focus on drug quality standards, ensures that pharmaceutical intermediates will remain the largest and most stable demand driver for etodolac methyl ester in the forecast period. Enhanced synthesis capabilities and improved quality performance continue to strengthen the segment's market position.
The etodolac methyl ester market is advancing steadily due to increasing pharmaceutical research initiatives and growing recognition of high-purity intermediate compound advantages over conventional synthesis materials. However, the market faces challenges including higher costs compared to lower-purity alternatives, need for specialized handling protocols for optimal utilization, and varying regulatory compliance requirements across different pharmaceutical jurisdictions. Technology advancement efforts and synthesis optimization programs continue to influence product development and market adoption patterns.
The growing development of advanced purification systems is enabling higher purity levels with improved synthesis consistency and reduced impurity characteristics. Enhanced purification technologies and optimized synthesis protocols provide superior intermediate performance while maintaining compliance with pharmaceutical manufacturing standards. These technologies are particularly valuable for pharmaceutical companies who require reliable intermediate performance that can support extensive manufacturing operations with consistent quality and synthesis reliability.
Modern etodolac methyl ester manufacturers are incorporating advanced quality assurance features and comprehensive documentation systems that enhance pharmaceutical compliance and product traceability. Integration of advanced quality control systems and optimized testing protocols enables superior synthesis results and comprehensive pharmaceutical manufacturing support. Advanced quality features support utilization in diverse pharmaceutical environments while meeting various synthesis requirements and regulatory specifications, including drug development, commercial manufacturing, and specialized pharmaceutical applications.
The continuous development of environmentally sustainable production technologies and green chemistry principles is reducing environmental impact while maintaining consistent intermediate quality throughout manufacturing processes. Advanced sustainability features and intelligent process optimization provide reduced environmental footprint capabilities that support comprehensive pharmaceutical manufacturing activities without quality compromise. These sustainability innovations are particularly valuable for companies conducting extensive pharmaceutical operations where environmental responsibility and consistent performance are essential for effective drug development and regulatory compliance.
| Country | CAGR (2025-2035) |
|---|---|
| China | 6.3% |
| India | 5.9% |
| Germany | 5.4% |
| Brazil | 4.9% |
| United States | 4.5% |
| United Kingdom | 4.0% |
| Japan | 3.5% |
The etodolac methyl ester market is growing rapidly, with China leading at a 6.3% CAGR through 2035, driven by strong pharmaceutical manufacturing expansion and increasing adoption of high-purity intermediate compounds. India follows at 5.9%, supported by rising pharmaceutical infrastructure development and growing awareness of advanced synthesis technologies. Germany grows steadily at 5.4%, integrating sophisticated intermediate compounds into its established pharmaceutical manufacturing infrastructure. Brazil records 4.9%, emphasizing pharmaceutical modernization and quality upgrade initiatives. The United States shows solid growth at 4.5%, focusing on synthesis enhancement and operational efficiency. The United Kingdom demonstrates steady progress at 4.0%, maintaining established pharmaceutical intermediate applications. Japan records 3.5% growth, concentrating on quality advancement and purity optimization.
The report covers an in-depth analysis of 40+ countries, the top-performing countries are highlighted below.
The etodolac methyl ester market in China is projected to exhibit the highest growth rate with a CAGR of 6.3% through 2035, driven by rapid expansion of pharmaceutical manufacturing infrastructure and increasing demand for high-purity intermediate compounds. The country's growing focus on pharmaceutical quality improvement and expanding drug production operations are creating significant demand for reliable synthesis materials. Major pharmaceutical manufacturers are establishing comprehensive supply networks to support the increasing requirements of drug development companies and pharmaceutical production facilities across research and commercial manufacturing applications. Government pharmaceutical development initiatives are supporting establishment of modern synthesis capabilities and advanced quality systems, driving demand for sophisticated intermediate compound technology throughout major pharmaceutical clusters and manufacturing corridors. Pharmaceutical manufacturing modernization programs are facilitating adoption of high-purity intermediate technology that enhances synthesis effectiveness and quality standards across production networks. The Chinese government's commitment to becoming a global pharmaceutical manufacturing hub and improving drug quality standards is accelerating investments in advanced intermediate compound procurement. Urban pharmaceutical development projects and biotechnology initiatives are incorporating sophisticated synthesis systems that require high-quality intermediate capabilities. Commercial drug manufacturing and research facility expansion programs are creating additional demand for pharmaceutical-grade intermediate compounds that meet international quality standards and synthesis reliability requirements.
The etodolac methyl ester market in India is expanding at a CAGR of 5.9%, supported by increasing pharmaceutical manufacturing development and growing awareness of high-purity intermediate compound benefits. The country's expanding pharmaceutical infrastructure and rising drug quality standards are driving demand for professional-grade synthesis materials. Pharmaceutical companies and contract manufacturing organizations are gradually implementing advanced intermediate compound technology to maintain synthesis standards and operational effectiveness. Pharmaceutical sector growth and manufacturing infrastructure development are creating opportunities for suppliers that can support diverse synthesis requirements and quality specifications. Professional training and quality assurance programs are building technical expertise among pharmaceutical personnel, enabling effective utilization of intermediate compound technology that meets pharmaceutical synthesis standards and quality requirements across metropolitan regions. India's rapid pharmaceutical expansion and increasing generic drug production are intensifying the need for effective synthesis management and quality intermediate capabilities. National pharmaceutical development programs and biotechnology park construction projects are establishing comprehensive synthesis systems that require advanced intermediate compounds. State government initiatives focusing on pharmaceutical manufacturing improvement and quality enhancement are driving procurement of modern intermediate compound technology for pharmaceutical companies. The growing emphasis on pharmaceutical quality standards and manufacturing excellence is supporting adoption of internationally certified synthesis materials.
The etodolac methyl ester market in Germany is projected to grow at a CAGR of 5.4%, supported by the country's emphasis on pharmaceutical quality standards and advanced synthesis technology adoption. German pharmaceutical companies are implementing sophisticated intermediate compound systems that meet stringent purity requirements and operational specifications. The market is characterized by focus on compound quality, synthesis reliability, and compliance with comprehensive pharmaceutical manufacturing standards. Pharmaceutical industry investments are prioritizing advanced intermediate technology that demonstrates superior synthesis performance and reliability while meeting German quality and operational standards. Professional certification programs are ensuring comprehensive technical expertise among pharmaceutical personnel, enabling specialized synthesis capabilities that support diverse pharmaceutical manufacturing applications and operational requirements across regional networks. Germany's commitment to maintaining the highest pharmaceutical manufacturing standards in Europe drives continuous investment in cutting-edge intermediate compound technology. The country's comprehensive pharmaceutical quality infrastructure requires sophisticated intermediate compounds that meet strict purity and performance standards. Integration with digital pharmaceutical systems and automated synthesis networks is creating demand for advanced intermediate compound technology with enhanced quality capabilities. Regional pharmaceutical companies are modernizing their synthesis capabilities through procurement of state-of-the-art intermediate compounds that support both research and commercial manufacturing operations.
The etodolac methyl ester market in Brazil is growing at a CAGR of 4.9%, driven by increasing pharmaceutical manufacturing development and growing recognition of high-purity intermediate compound advantages. The country's expanding pharmaceutical production system is gradually integrating professional-grade intermediate compound technology to enhance synthesis capabilities and operational effectiveness. Pharmaceutical companies and manufacturing facilities are investing in intermediate compound technology to address evolving drug quality requirements and synthesis standards. Manufacturing modernization is facilitating adoption of advanced synthesis technologies that support comprehensive quality capabilities across pharmaceutical production regions. Professional development programs are enhancing technical capabilities among pharmaceutical personnel, enabling effective intermediate compound utilization that meets evolving drug quality standards and operational requirements throughout manufacturing networks. Brazil's extensive pharmaceutical market and growing domestic drug production sector are driving demand for sophisticated synthesis intermediate equipment. Federal and state government initiatives focused on improving pharmaceutical quality and expanding manufacturing capabilities are supporting procurement of modern intermediate compound technology. Urban pharmaceutical development and increasing drug manufacturing capacity in major metropolitan areas require enhanced synthesis capabilities that can support effective production operations. The expansion of professional pharmaceutical services and specialized manufacturing facilities is creating sustained demand for reliable, high-purity intermediate compounds.
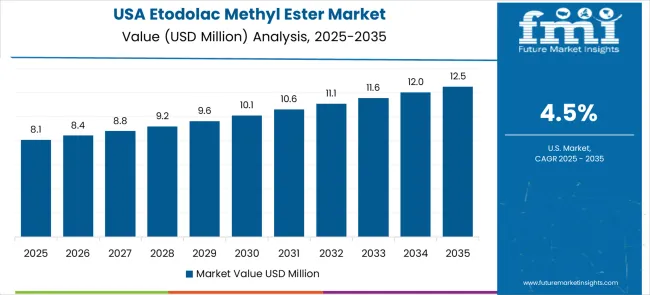
The etodolac methyl ester market in the USA is expanding at a CAGR of 4.5%, driven by established pharmaceutical companies and growing emphasis on synthesis efficiency enhancement. Large pharmaceutical manufacturers and contract development organizations are implementing comprehensive intermediate compound capabilities to serve diverse synthesis requirements. The market benefits from established supply distribution networks and professional quality programs that support various pharmaceutical manufacturing applications. Pharmaceutical industry leadership is enabling standardized intermediate compound utilization across multiple synthesis types, providing consistent quality performance and comprehensive operational coverage throughout regional markets. Professional development and certification programs are building specialized technical expertise among pharmaceutical personnel, enabling effective intermediate compound utilization that supports evolving synthesis requirements across metropolitan areas. The USA market is characterized by continuous technology advancement and integration with modern pharmaceutical manufacturing systems. Federal drug development programs are driving procurement of sophisticated intermediate compound equipment that meets stringent purity and reliability standards. Pharmaceutical companies and biotechnology organizations are modernizing their synthesis capabilities through adoption of advanced intermediate compound technology. The emphasis on manufacturing efficiency and quality assurance is supporting demand for high-performance, reliable intermediate compound designs that enhance synthesis effectiveness while maintaining quality standards.
The etodolac methyl ester market in the UK is projected to grow at a CAGR of 4.0%, supported by established pharmaceutical sectors and growing emphasis on synthesis capabilities. British pharmaceutical companies and manufacturing services are implementing intermediate compound equipment that meets industry quality standards and operational requirements. The market benefits from established pharmaceutical manufacturing infrastructure and comprehensive training programs for synthesis professionals. Pharmaceutical quality investments are prioritizing advanced intermediate compounds that support diverse manufacturing applications while maintaining established quality and operational standards. Professional development programs are building technical expertise among pharmaceutical personnel, enabling specialized intermediate compound operation capabilities that meet evolving synthesis requirements and quality standards throughout regional manufacturing networks. The United Kingdom's comprehensive pharmaceutical strategy and commitment to maintaining drug quality drives continued investment in advanced synthesis technology. Pharmaceutical companies across England, Wales, Scotland, and Northern Ireland are standardizing their intermediate compound equipment to ensure consistent synthesis capabilities. Integration with national pharmaceutical monitoring systems and coordination with regulatory compliance programs requires sophisticated intermediate compound technology. The emphasis on professional pharmaceutical training and equipment certification is supporting adoption of high-quality synthesis materials that meet MHRA approval standards and operational requirements.
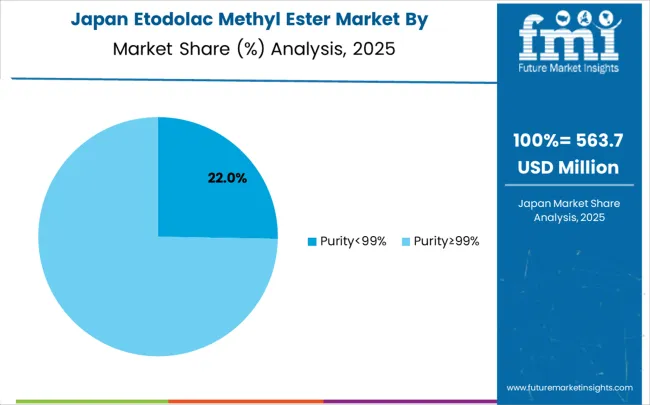
The etodolac methyl ester market in Japan is growing at a CAGR of 3.5%, driven by the country's focus on synthesis technology innovation and quality enhancement applications. Japanese pharmaceutical companies are implementing advanced intermediate compound systems that demonstrate superior purity reliability and synthesis efficiency. The market is characterized by emphasis on technological excellence, quality assurance, and integration with established pharmaceutical manufacturing workflows. Technology industry investments are prioritizing innovative synthesis solutions that combine advanced intermediate compound technology with precision quality control while maintaining Japanese quality and reliability standards. Professional development programs are ensuring comprehensive technical expertise among pharmaceutical personnel, enabling specialized synthesis capabilities that support diverse pharmaceutical manufacturing applications and operational requirements throughout metropolitan pharmaceutical networks. Japan's commitment to maintaining the highest standards of pharmaceutical quality and manufacturing drives continuous innovation in intermediate compound technology. The integration of intermediate compound systems with intelligent pharmaceutical infrastructure and automated synthesis networks requires sophisticated compound capabilities. Pharmaceutical companies across Japan's regions are modernizing their synthesis equipment through procurement of cutting-edge intermediate compound technology that meets strict performance and reliability standards. The emphasis on precision quality control and technological advancement is supporting development of next-generation synthesis systems with enhanced purity and operational capabilities.
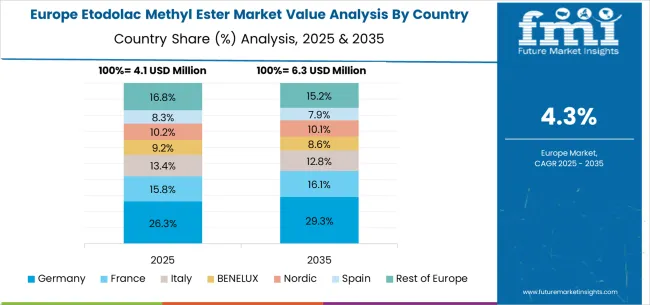
The etodolac methyl ester market in Europe is anticipated to expand from USD 4.9 million in 2025 to approximately USD 7.8 million by 2035, reflecting a CAGR of 4.7% during the forecast period. Germany is set to reinforce its leading position, commanding a 32.1% share of the European market in 2025, supported by its established pharmaceutical manufacturing infrastructure, high standards for synthesis quality, and continuous investment in advanced intermediate compound technologies. The United Kingdom follows with a market share of 19.4%, benefiting from standardized pharmaceutical procedures, professional training programs, and a robust focus on drug quality modernization. France is projected to capture 16.8% of the regional market, fueled by steady integration of modern synthesis intermediate equipment and enhancements in pharmaceutical manufacturing operations. Italy and Spain together represent 20.2% of total demand, as both countries advance their pharmaceutical manufacturing modernization efforts and invest in professional-grade synthesis solutions for drug manufacturers. The Rest of Europe, comprising Nordic countries, BENELUX, and Eastern European markets, accounts for 11.5%, driven by regulatory convergence, infrastructure upgrading, and broader adoption of advanced etodolac methyl ester technology to support evolving pharmaceutical quality requirements and synthesis programs.
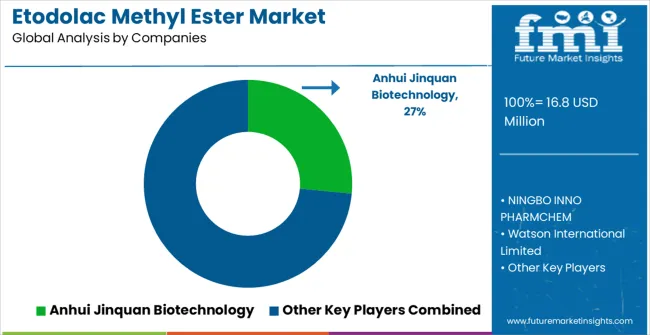
The etodolac methyl ester market is composed of 10–15 specialized manufacturers, with the top five companies controlling 60–65% of global market share, driven by demand for pharmaceutical intermediates, anti-inflammatory drugs, and analgesic formulations. Competition focuses on purity, regulatory compliance, cost-effectiveness, and supply chain reliability, rather than price alone. Anhui Jinquan Biotechnology leads the market with an 27% share, supported by its strong position in producing high-purity etodolac derivatives and its comprehensive pharmaceutical-grade chemical offerings.
Other major players such as NINGBO INNO PHARMCHEM, Watson International Limited, and Hunan Huateng Pharmaceutical maintain strong market positions through high-quality production processes, global distribution channels, and well-established relationships with pharmaceutical manufacturers. These companies leverage their expertise in chemical synthesis, process optimization, and regulatory approvals to meet the growing demand for etodolac methyl ester as a key precursor in anti-inflammatory formulations.
Challengers like Shanghai Haohong Scientific, Jinan Haohua Industry, and Hebei Chengxin focus on providing cost-effective etodolac methyl ester solutions, particularly for bulk production and generic pharmaceutical manufacturing. Regional players such as Zhejiang Ausun Pharmaceutical, Changzhou Highassay Chemical, and Biosynth Carbosynth strengthen their presence by offering high-purity, custom-synthesized compounds and building strong local supply networks. Alfa Chemistry, ChemScene, Toronto Research Chemicals, and Sigma-Aldrich (Merck) further diversify the competitive landscape by offering specialized intermediates and custom synthesis services to cater to research institutions and pharmaceutical R&D segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 16.8 million |
| Purity Level | Purity <99%, Purity ≥99% |
| Application | Pharmaceutical Intermediates, Research Applications |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | Anhui Jinquan Biotechnology, NINGBO INNO PHARMCHEM, Watson International Limited, Hunan Huateng Pharmaceutical, Shanghai Haohong Scientific, Jinan Haohua Industry, Hebei Chengxin, Zhejiang Ausun Pharmaceutical, Changzhou Highassay Chemical, Biosynth Carbosynth, Alfa Chemistry, ChemScene, Toronto Research Chemicals, and Sigma-Aldrich (Merck) |
| Additional Attributes | Dollar sales by purity level and application segment, regional demand trends across major markets, competitive landscape with established intermediate manufacturers and emerging synthesis providers, customer preferences for different purity grades and synthesis options, integration with pharmaceutical manufacturing systems and quality protocols, innovations in purification technologies and synthesis optimization methods, and adoption of sustainable manufacturing features with enhanced quality capabilities for improved pharmaceutical workflows. |
The global etodolac methyl ester market is estimated to be valued at USD 16.8 million in 2025.
The market size for the etodolac methyl ester market is projected to reach USD 26.7 million by 2035.
The etodolac methyl ester market is expected to grow at a 4.7% CAGR between 2025 and 2035.
The key product types in etodolac methyl ester market are purity<99% and purity≥99%.
In terms of application, pharmaceutical intermediates segment to command 78.0% share in the etodolac methyl ester market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Fatty Methyl Ester Sulfonate Market Size and Share Forecast Outlook 2025 to 2035
3,3-Dimethylacrylic Acid Methyl Ester Market Size and Share Forecast Outlook 2025 to 2035
Methylcobalamin Market Size and Share Forecast Outlook 2025 to 2035
Methylparaben Market Forecast and Outlook 2025 to 2035
Methyl Cyclohexane Market Size and Share Forecast Outlook 2025 to 2035
Methyl 3-Oxovalerate Market Size and Share Forecast Outlook 2025 to 2035
Methyl 2-Fluoro-3-Oxopentanoate Market Size and Share Forecast Outlook 2025 to 2035
Methyl 3-Methyl-2-Butenoate Market Size and Share Forecast Outlook 2025 to 2035
Methyl 2-Naphthyl Ether Market Size and Share Forecast Outlook 2025 to 2035
Esterquats Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ionone Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ethyl Ketone Market Size and Share Forecast Outlook 2025 to 2035
Methyl Isobutyl Carbinol Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ethyl Ketone Peroxide (MEKP) Market Size and Share Forecast Outlook 2025 to 2035
Esterified Vitamins Market Size, Growth, and Forecast for 2025 to 2035
Methyl Oleate Market – Trends & Forecast 2025 to 2035
Esters Market Trends & Outlook 2025 to 2035
Ester Gums Market Growth – Trends & Forecast 2025 to 2035
Methylamine Market Growth – Trends & Forecast 2024-2034
Methyl p-hydroxybenzoate Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA