The mycobacterium tuberculosis PCR detection kits market is expected to grow at USD 616.4 million in 2025 and expected to reach USD 985.1 million by 2035, advancing at a CAGR of 4.8%. Inflection point mapping provides a structured view of how growth dynamics shift over the timeline. From 2020 to 2025, revenue rises from USD 487.6 million to USD 616.4 million, showing the first inflection point. This stage highlights steady demand growth driven by heightened awareness, diagnostic capacity improvements, and increased global initiatives for tuberculosis testing. It represents the initial acceleration period where adoption broadens from large healthcare institutions into broader regional programs.
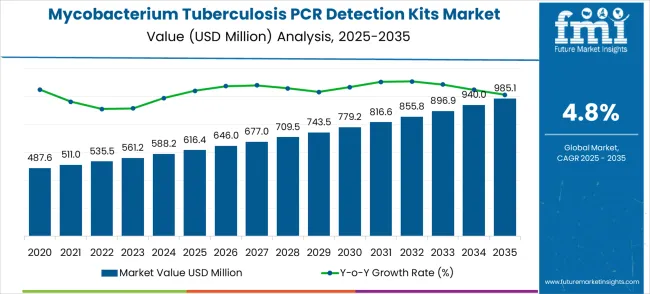
| Metric | Value |
|---|---|
| Market Value (2025) | USD 616.4 million |
| Market Forecast Value (2035) | USD 985.1 million |
| Forecast CAGR (2025-2035) | 4.8% |
Between 2025 and 2030, revenue climbs from USD 616.4 million to USD 779.2 million, indicating the second inflection point. Growth during this period reflects rising integration of PCR detection kits into public health systems and community-level diagnostic networks. The pace of expansion is influenced by government-led tuberculosis eradication programs and partnerships between diagnostic companies and NGOs. Inflection mapping here shows the transition from selective adoption to a more mainstream role in diagnostic protocols, with demand spreading across both developed and developing healthcare markets.
From 2030 to 2035, revenue expands from USD 779.2 million to USD 985.1 million, reflecting the third key inflection point. This phase represents the market’s movement toward maturity, where adoption is widespread, and incremental growth is driven by test optimization, higher accessibility in remote regions, and replacement demand. Inflection mapping during this period indicates a slower but stable upward curve, highlighting that while large-scale expansion slows, the market continues to grow steadily due to global tuberculosis prevalence. By 2035, the market is positioned in the late-growth phase, where reliability, cost-effectiveness, and integration with molecular diagnostics platforms become the defining growth enablers.
Market expansion is being supported by revolutionary advances in molecular diagnostics technology and tuberculosis surveillance automation, creating unprecedented opportunities for rapid pathogen detection and comprehensive disease monitoring optimization. Modern healthcare systems increasingly demand sophisticated PCR detection systems that enable precise tuberculosis identification, support drug resistance testing, and provide reliable diagnostic capabilities for clinical decision-making and public health surveillance. The integration of advanced molecular technology with comprehensive diagnostic workflows enables previously impossible levels of tuberculosis detection accuracy and clinical diagnostic efficiency.
The growing focus on global tuberculosis elimination and diagnostic standardization is driving massive demand for Mycobacterium tuberculosis PCR detection kits from leading healthcare institutions with proven track records of innovation and diagnostic excellence. Healthcare systems and diagnostic laboratories are investing significantly in molecular diagnostic technologies that offer superior detection sensitivity while providing enhanced diagnostic speed and comprehensive tuberculosis monitoring through advanced PCR testing protocols. Diagnostic standards are establishing performance benchmarks that favor automated PCR systems with advanced detection capabilities and superior clinical diagnostic integration features.
The healthcare industry's transformation toward molecular diagnostic automation is creating substantial demand for intelligent PCR detection products capable of delivering professional-grade tuberculosis diagnostics and disease surveillance through advanced molecular testing platforms. The public health sector continues to drive innovation in tuberculosis control applications while maintaining diagnostic accuracy and operational efficiency, leading to development of breakthrough PCR detection systems with enhanced sensitivity capabilities and comprehensive tuberculosis surveillance integration.
The market is segmented by product type, application, and region. By product type, the market is divided into conventional PCR kits, real-time PCR kits, and other configurations. Based on application, the market is categorized into clinical diagnosis, epidemiological surveys, treatment monitoring, and other applications. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
Real-time PCR kits configurations are projected to account for 64% of the Mycobacterium tuberculosis PCR detection kits market in 2025. This leading share is supported by explosive adoption of rapid diagnostic requirements and growing demand for quantitative tuberculosis detection through advanced molecular technology. Real-time PCR kits provide unprecedented detection speed and quantitative analysis capabilities, making them the preferred choice for clinical laboratories seeking comprehensive tuberculosis diagnostics and enhanced diagnostic workflow efficiency. The segment benefits from revolutionary advances in real-time detection technology, automated analysis systems, and integrated diagnostic platforms that have created entirely new categories of rapid molecular diagnostic devices.
Modern real-time PCR kits incorporate sophisticated detection algorithms and advanced molecular analysis systems that enable real-time pathogen quantification, rapid result generation, and seamless integration with laboratory information systems while ensuring superior diagnostic accuracy and comprehensive clinical workflow integration. These innovations have transformed tuberculosis diagnostics while providing superior detection capabilities for clinical decision-making and treatment monitoring through continuous molecular analysis and intelligent diagnostic processing. The clinical diagnostics market particularly drives demand for real-time PCR solutions, as these applications require rapid result generation and reliable detection accuracy to deliver superior diagnostic outcomes and clinical care optimization.
The hospital and reference laboratory sectors increasingly adopt advanced real-time PCR technologies to support tuberculosis diagnosis and monitor treatment response through precise molecular detection and comprehensive clinical assessment. The growing emphasis on diagnostic speed creates opportunities for specialized real-time PCR systems designed for critical clinical applications and comprehensive tuberculosis diagnostic management.
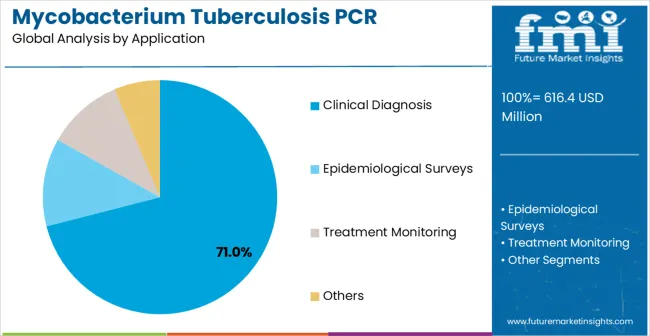
Clinical diagnosis applications are expected to represent 71% of Mycobacterium tuberculosis PCR detection kits demand in 2025. This dominant share reflects the massive healthcare market for tuberculosis diagnostics and growing investment in molecular diagnostic technologies for comprehensive clinical assessment. Clinical diagnosis applications enable comprehensive tuberculosis detection through sophisticated molecular testing, automated diagnostic workflows, and advanced clinical decision support systems. The segment benefits from breakthrough advances in clinical molecular diagnostics that makes sophisticated tuberculosis detection accessible and critical for clinical accuracy and patient care optimization.
The global healthcare revolution drives significant demand for clinical tuberculosis diagnostic systems that provide exceptional detection capabilities and intelligent diagnostic integration for clinical care and patient management applications. These applications require PCR detection systems with superior diagnostic abilities and comprehensive clinical integration to ensure reliable tuberculosis detection and effective clinical decision-making capabilities. The segment benefits from growing healthcare investment in molecular diagnostic technologies and increasing implementation of automated clinical testing systems and comprehensive diagnostic solutions.
Healthcare and clinical laboratory sectors contribute substantially to market growth as institutions implement PCR detection systems in clinical workflows and comprehensive patient care strategies. The growing adoption of molecular diagnostics creates opportunities for specialized clinical tuberculosis detection systems designed for healthcare applications and comprehensive clinical diagnostic management. Additionally, the trend toward diagnostic automation drives demand for intelligent clinical PCR systems that enable comprehensive tuberculosis detection through integrated molecular testing, clinical decision support, and patient care management capabilities.
The Mycobacterium tuberculosis PCR detection kits market is advancing steadily due to revolutionary molecular diagnostics development and unprecedented adoption of tuberculosis surveillance technologies across all healthcare and public health segments. However, the market faces challenges including high reagent costs, need for robust quality control validation, and varying regulatory requirements across different diagnostic applications. Diagnostic accuracy standards and clinical laboratory regulations continue to influence product development and market adoption patterns.
The revolutionary advances in PCR amplification systems, molecular detection algorithms, and automated laboratory technologies are enabling unprecedented tuberculosis diagnostic capabilities while maintaining clinical accuracy and operational efficiency standards. Advanced molecular architectures and diagnostics-specific detection systems provide superior sensitivity and diagnostic accuracy capabilities, enabling dynamic clinical optimization and enhanced tuberculosis detection reliability. These technologies are particularly valuable for clinical applications that require sophisticated molecular detection, accurate pathogen identification, and reliable diagnostic standardization protocols.
Modern Mycobacterium tuberculosis PCR detection kit manufacturers are implementing breakthrough ecosystem integration and comprehensive diagnostic platform development that enables seamless laboratory integration while supporting holistic tuberculosis monitoring and clinical workflow enhancement. Advanced platform architectures enable unified molecular testing, diagnostic reporting, and clinical management while ensuring diagnostic accuracy compliance and laboratory operational requirements.
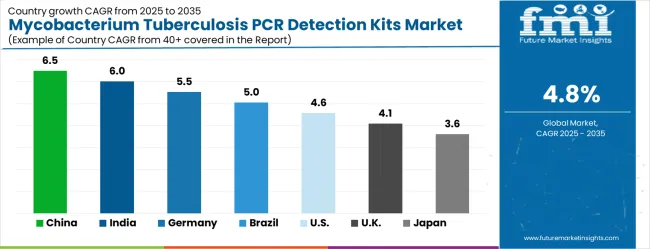
| Country | CAGR (2025-2035) |
|---|---|
| China | 6.5% |
| India | 6.0% |
| Germany | 5.5% |
| Brazil | 5.0% |
| United States | 4.6% |
| United Kingdom | 4.1% |
| Japan | 3.6% |
The mycobacterium tuberculosis PCR detection kits market is experiencing moderate growth, with China leading at 6.5% CAGR through 2035, driven by massive healthcare infrastructure investment and rapidly expanding tuberculosis surveillance programs. India follows at 6.0%, supported by enormous tuberculosis burden potential and increasing molecular diagnostics accessibility investment. Germany records solid growth at 5.5%, emphasizing diagnostic technology innovation excellence and advanced molecular testing development. Brazil grows steadily at 5.0%, benefiting from expanding healthcare infrastructure and tuberculosis control programs. The United States shows consistent growth at 4.6%, focusing on diagnostic technology advancement and clinical molecular testing applications. The United Kingdom maintains steady expansion at 4.1%, supported by healthcare innovation development. Japan demonstrates stable growth at 3.6%, emphasizing precision diagnostic technology and clinical excellence.
The report covers an in-depth analysis of 40+ countries top-performing countries are highlighted below.
Mycobacterium tuberculosis PCR detection kits in China is projected to exhibit the highest growth rate with a CAGR of 6.5% through 2035, driven by the country's position as a healthcare infrastructure powerhouse and massive domestic investment in tuberculosis control program development. The extensive healthcare modernization and unprecedented investment in molecular diagnostics technology are creating extraordinary opportunities for PCR detection kit adoption. Major healthcare and diagnostic companies are developing world-leading tuberculosis detection capabilities to serve both the enormous domestic healthcare market and global diagnostic expansion while establishing leadership in molecular diagnostic applications.
Revenue from mycobacterium tuberculosis PCR detection kits in India is expanding at a CAGR of 6.0%, supported by the country's massive tuberculosis burden potential and rapidly accelerating healthcare infrastructure across all regions and clinical segments. The enormous tuberculosis control program expansion and increasing molecular diagnostics adoption are driving extraordinary PCR detection kit demand potential. Healthcare technology companies are leveraging India's vast tuberculosis burden while developing cost-effective diagnostic solutions to capture emerging opportunities in clinical diagnosis and public health surveillance applications.
Mycobacterium tuberculosis PCR detection kits in Germany is projected to grow at a CAGR of 5.5%, supported by the country's leadership in diagnostic technology innovation and advanced molecular testing development across healthcare, pharmaceutical, and biotechnology sectors. German diagnostic technology companies are implementing sophisticated PCR detection development that meets stringent regulatory standards and clinical requirements while delivering superior diagnostic properties and system reliability. The market is characterized by focus on healthcare innovation, advanced technology integration, and compliance with comprehensive diagnostic accuracy and clinical quality regulations.
Revenue from mycobacterium tuberculosis PCR detection kits in Brazil is growing at a CAGR of 5.0%, driven by expanding healthcare infrastructure and increasing tuberculosis control investment across urban and emerging healthcare market segments. The growing healthcare awareness and increasing diagnostic infrastructure development are creating substantial opportunities for PCR detection kit market development. Healthcare technology companies are adapting molecular diagnostic systems to support growing tuberculosis control demand and healthcare development while maintaining cost accessibility and local market relevance.
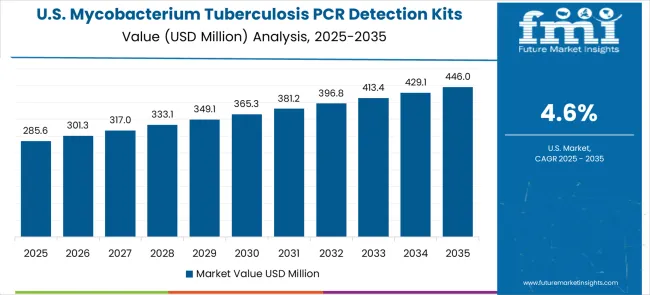
Demand for mycobacterium tuberculosis PCR detection kits in the United States is expanding at a CAGR of 4.6%, driven by the country's diagnostic technology advancement and healthcare market demand for advanced molecular diagnostic products and cutting-edge clinical testing technologies. The sophisticated healthcare infrastructure and clinical willingness to invest in advanced PCR detection systems create significant demand for high-performance diagnostic solutions. The market benefits from breakthrough molecular diagnostic research and healthcare-grade technology market segments across clinical, reference laboratory, and public health applications.
Mycobacterium tuberculosis PCR detection kits in the United Kingdom is projected to grow at a CAGR of 4.1%, supported by ongoing healthcare innovation advancement and increasing clinical demand for intelligent molecular diagnostic products in clinical and public health applications. Molecular diagnostic technology companies are investing in healthcare technology development that provides advanced functionality while meeting regulatory requirements and clinical compliance for healthcare applications. The market is characterized by focus on healthcare innovation, diagnostic accuracy protection, and advanced technology integration across diverse clinical segments.
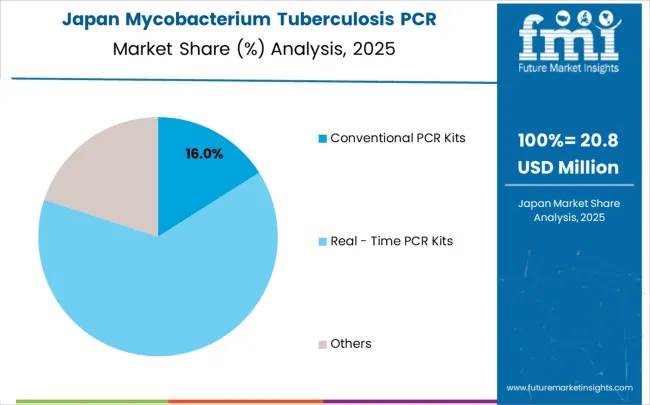
Demand for mycobacterium tuberculosis PCR detection kits in Japan is expanding at a CAGR of 3.6%, driven by the country's emphasis on diagnostic technology excellence and precision clinical development across healthcare, electronics, and biotechnology sectors. Japanese diagnostic technology companies are developing sophisticated PCR detection applications that incorporate precision engineering and clinical optimization principles. The market benefits from focus on diagnostic precision, technology reliability, and continuous innovation in clinical technology and healthcare diagnostic integration.
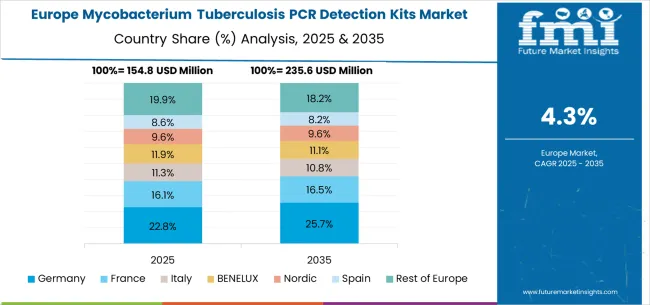
The mycobacterium tuberculosis PCR detection kits market in Europe is projected to grow from USD 165.1 million in 2025 to USD 263.9 million by 2035, registering a CAGR of 4.8% over the forecast period. Germany is expected to maintain its leadership with a 33.7% share in 2025, supported by its advanced healthcare technology industry and molecular diagnostics excellence. The United Kingdom follows with 24.2% market share, driven by healthcare innovation advancement and diagnostic technology development. France holds 18.8% of the European market, benefiting from healthcare technology expansion and molecular diagnostics industry development. Italy and Spain collectively represent 14.6% of regional demand, with growing focus on clinical diagnostics development and molecular testing applications. The Rest of Europe region accounts for 8.7% of the market, supported by diagnostic technology development in Eastern European countries and Nordic healthcare innovation advancement.
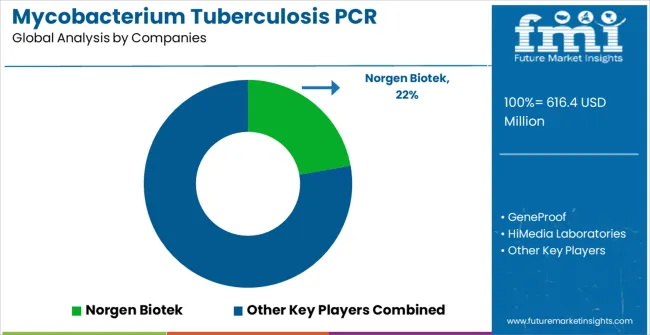
The mycobacterium tuberculosis PCR detection kits market is defined by steady competition among molecular diagnostic manufacturers, innovative healthcare technology companies, and established clinical laboratory suppliers. Companies are investing in molecular diagnostic development, advanced kit design, diagnostic accuracy validation, and clinical ecosystem integration to deliver reliable, sensitive, and healthcare-compatible PCR detection products. Strategic partnerships, technological advancement, and consistent healthcare market expansion are central to achieving market leadership and clinical adoption.
Norgen Biotek, operating globally, offers comprehensive molecular diagnostic solutions with focus on clinical and research applications, diagnostic performance optimization, and seamless laboratory integration across PCR kits, extraction systems, and diagnostic services applications. GeneProof, technology provider, delivers advanced molecular diagnostic systems with emphasis on clinical reliability, diagnostic performance, and intelligent laboratory integration capabilities. HiMedia Laboratories provides innovative diagnostic solutions with focus on comprehensive clinical integration and high-performance molecular applications. QIAGEN offers precision molecular diagnostic systems with advanced PCR technology and clinical-grade performance capabilities.
CerTest Biotec provides specialized molecular diagnostic systems with emphasis on clinical and research applications. Daan Gene Co., Ltd. delivers advanced molecular diagnostic technology with sophisticated clinical performance and laboratory optimization capabilities. Zeesan Biotech offers comprehensive diagnostic solutions with clinical integration and molecular diagnostic services. Zhejiang Orient Gene Biotech Co., Ltd provides diagnostic systems with advanced clinical integration and molecular diagnostic systems.
Hangzhou Bioer Technology, Sacace Biotechnologies, Solgent, INtRON Biotechnology, Primerdesign, ELITechGroup, Bioneer, Fosun Pharma, Sansure Biotech, NZYTech, BioSellal, Genmark Saglik Urunleri, and Mylab Discovery Solutions offer specialized molecular diagnostic expertise, innovative product development, and technical advancement across PCR detection and clinical diagnostic technology networks.
The mycobacterium tuberculosis PCR detection kits market underpins global tuberculosis control revolution, molecular diagnostics advancement, healthcare technology democratization, and clinical diagnostic optimization evolution. With accelerating tuberculosis surveillance, molecular diagnostic expansion, and clinical accuracy requirements, the sector faces pressure to balance innovation velocity, diagnostic quality protection, and clinical performance validation. Coordinated contributions from governments, industry bodies, OEMs/technology integrators, suppliers, and investors will accelerate the transition toward beneficial, accurate, and universally accessible Mycobacterium tuberculosis PCR detection kit ecosystems.
| Item | Value |
|---|---|
| Quantitative Units | USD 616.4 million |
| Product Type | Conventional PCR Kits, Real-Time PCR Kits, Others |
| Application | Clinical Diagnosis, Epidemiological Surveys, Treatment Monitoring, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, India, China, United Kingdom, Japan, Brazil, and other 40+ countries |
| Key Companies Profiled | Norgen Biotek, GeneProof, HiMedia Laboratories, QIAGEN, CerTest Biotec, Daan Gene Co., Ltd., Zeesan Biotech, Zhejiang Orient Gene Biotech Co., Ltd, Hangzhou Bioer Technology, Sacace Biotechnologies, Solgent, INtRON Biotechnology, Primerdesign, ELITechGroup, Bioneer, Fosun Pharma, Sansure Biotech, NZYTech, BioSellal, Genmark Saglik Urunleri, Mylab Discovery Solutions |
The global mycobacterium tuberculosis PCR detection kits market is estimated to be valued at USD 616.4 million in 2025.
The market size for the mycobacterium tuberculosis PCR detection kits market is projected to reach USD 985.1 million by 2035.
The mycobacterium tuberculosis PCR detection kits market is expected to grow at a 4.8% CAGR between 2025 and 2035.
The key product types in mycobacterium tuberculosis PCR detection kits market are conventional PCR kits, real - time PCR kits and others.
In terms of application, clinical diagnosis segment to command 71.0% share in the mycobacterium tuberculosis PCR detection kits market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Mycobacterium Tuberculosis Testing Market
Nontuberculous Mycobacterium Treatment Market
Tuberculosis Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Tuberculosis Diagnostics Market
Ocular Tuberculosis Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Drug Resistant Pulmonary Tuberculosis Market
Hip Kits Market Size and Share Forecast Outlook 2025 to 2035
Oiler Kits Market Size and Share Forecast Outlook 2025 to 2035
Audio Kits Market
Lavage Kits Market
Organoids Kits Market
Capacitor Kits Market
Connector Kits Market
Extraction Kits Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
DIY Haircut Kits Market - Trends, Growth & Forecast 2025 to 2035
EMI, Filter Kits Market
Nephelometry Kits Market Size and Share Forecast Outlook 2025 to 2035
Analysis and Growth Projections for Chilled Meal Kits Market
Demining Tool Kits Market Size and Share Forecast Outlook 2025 to 2035
Antibody Pair Kits Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA