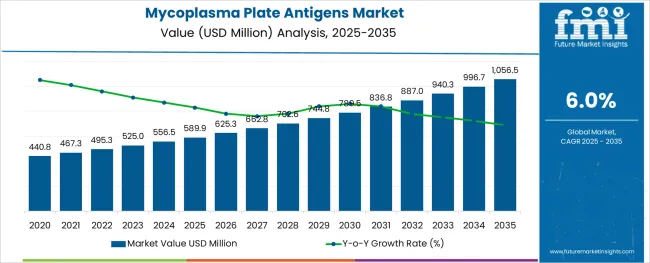
The Mycoplasma Plate Antigens Market is estimated to be valued at USD 589.9 million in 2025 and is projected to reach USD 1056.5 million by 2035, registering a compound annual growth rate (CAGR) of 6.0% over the forecast period.
The Mycoplasma plate antigens market is growing steadily as the need for reliable diagnostic tests in both human and veterinary health increases. The rising focus on disease control and drug development has enhanced demand for accurate antigen test products that detect mycoplasma infections. Advances in diagnostic methodologies have improved the sensitivity and specificity of plate antigen tests, enabling earlier and more reliable detection.
Additionally, veterinary healthcare settings have expanded their use of mycoplasma testing to manage infections in livestock and pets, which has contributed significantly to market growth. Government health initiatives and increasing awareness about zoonotic diseases have also played a role in driving adoption.
The market outlook is positive, with innovations expected in antigen preparation and assay techniques that can support faster and more cost-effective testing. Segmental growth is expected to be led by Plate Agglutination Mycoplasma Plate Antigen Test Products as the dominant product type, applications in drug development, and veterinary hospitals as a key user segment.
The market is segmented by Product Type, Application Type, and User Type and region. By Product Type, the market is divided into Plate Agglutination Mycoplasma Plate Antigen Test Products, Mycoplasma Plate Antigens, Mycoplasma Plate Antigens- Control Serum, Plate Antigens-Negative Control Serum, Confirmatory Testing Reagents, Mycoplasma Whole Organism, Mycoplasma Antiserum, and Negative Serum. In terms of Application Type, the market is classified into For Drug Development, For Infection Medicine, For Veterinary Research, and For Other Applications. Based on User Type, the market is segmented into For Veterinary Hospitals, For Poultry Veterinary Clinics, For Academic and Research Institutes, and For Pharma and Biotech Companies. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
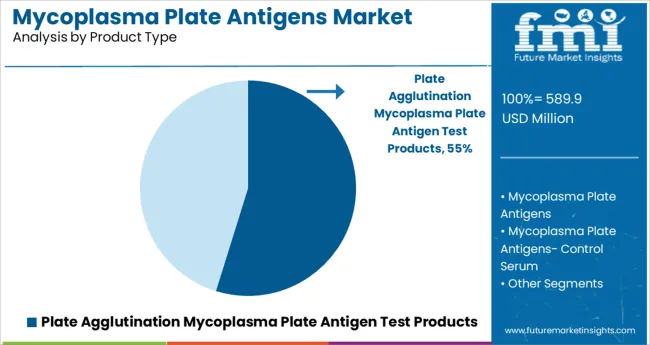
The Plate Agglutination Mycoplasma Plate Antigen Test Products segment is projected to hold 54.8% of the market revenue in 2025, securing its position as the leading product type. This segment’s growth is attributed to the widespread adoption of plate agglutination tests for their reliability in detecting mycoplasma antibodies.
The simplicity and cost-effectiveness of this method have made it a preferred choice in both research and clinical diagnostic settings. The ability to provide rapid results with minimal specialized equipment has further driven its use, particularly in veterinary hospitals and drug development laboratories.
Continuous improvements in antigen purity and assay sensitivity are expected to sustain the segment’s dominance as the market evolves.
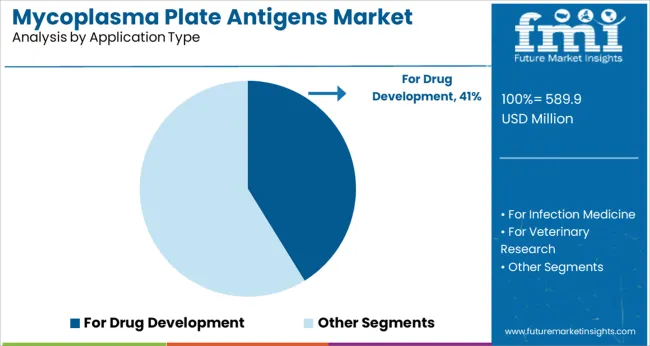
The For Drug Development application segment is projected to contribute 41.2% of the market revenue in 2025, maintaining its leading role. Drug development processes require stringent testing for contamination including mycoplasma to ensure safety and efficacy of biological products.
Plate antigen tests have become integral in preclinical and clinical phases for monitoring contamination. The emphasis on quality control and regulatory compliance has propelled the adoption of these tests in pharmaceutical research.
As biologics and cell therapy products continue to expand, the demand for reliable mycoplasma detection methods in drug development is expected to rise further.
The Veterinary Hospitals user segment is expected to account for 36.7% of the market revenue in 2025, positioning it as a major user type. Veterinary healthcare providers have increased the use of mycoplasma antigen tests to manage infectious diseases in animals, which are crucial for animal welfare and public health.
The prevalence of respiratory and reproductive mycoplasma infections in livestock and companion animals has driven routine screening and diagnosis. Veterinary hospitals benefit from the availability of quick and accurate plate agglutination tests which aid in timely treatment decisions.
Growing awareness about zoonotic risks and animal health regulations further support the sustained demand from this segment.
Mycoplasma infection can occur in humans and other animals, usually as a result of close contact with infected poultry. In humans, these infections are associated with mild respiratory disease and can cause mild to severe illness. As a result, diagnosing mycoplasma plate antigens has become a common practice.
Many biochemical and physiological properties of some reagents and chemicals are usually insufficient for strain identification and classification based on the order mycoplasmatales. As a result, the serological properties of mycoplasma plate antigens are widely used for such purposes.
In comparison to other available reagents, these mycoplasma plate antigens provide more accurate quantification of antibodies against mycoplasma. These serological reactions are successfully used to identify antibodies against mycoplasma; however, research is still being conducted to determine the various cell components responsible for these reactions.
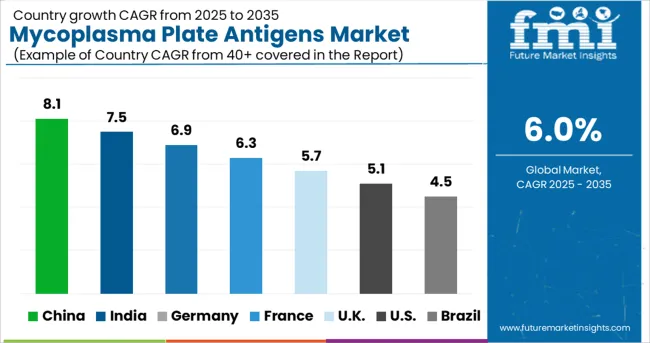
China and India are expected to grow at a steady rate in the Asian mycoplasma plate antigens market over the forecast period. Factors such as a significant increase in import reforms for medical and life science products, as well as the presence of local manufacturers with R&D facilities in the region, are expected to drive the growth of the Mycoplasma Plate Antigens market in the region.
Among other countries in the region, Japan, which has established R&D in medical devices as well as developed healthcare infrastructure, will contribute to the country's market growth for mycoplasma plate antigens.
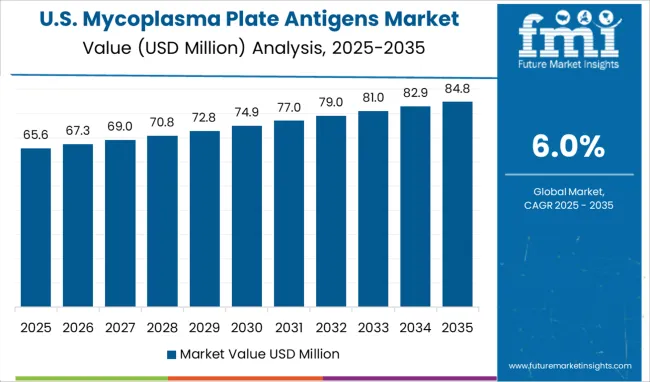
Geographically, the global Mycoplasma Plate Antigens market is divided into the following key regions: North America, Latin America, Europe, South Asia, East Asia, Oceania, and the Middle East and Africa. In the forecasted period, North America is expected to account for one of the largest shares of the global mycoplasma plate antigens market. Increased drug development, infection medicine, veterinary research, and other cell-based studies are among the drivers for the region of North America.
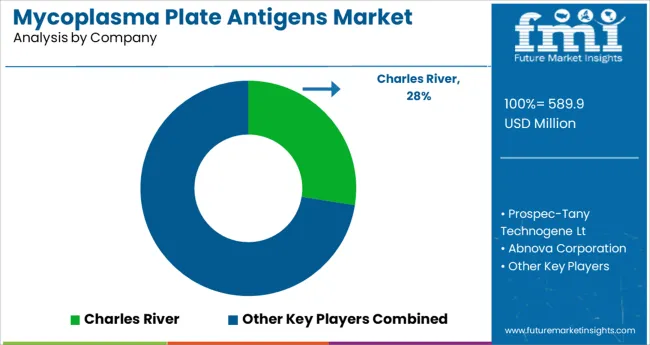
Some of the key players present in global mycoplasma plate antigens market include Charles River, Prospec-Tany Technogene Lt, Abnova Corporation, Qiagen N.V, and MyBiosource, Inc.among others.
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 6% until 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in billion, Volume in Kilotons and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered |
Product Type, Application Type, End User Type, Region |
| Regions Covered |
North America; Latin America; Western Europe; Eastern Europe; APEJ; Japan; Middle East and Africa |
| Key Countries Profiled |
USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, Nordics, BENELUX, Australia & New Zealand, China, India, ASEAN, GCC, South Africa |
The global mycoplasma plate antigens market is estimated to be valued at USD 589.9 million in 2025.
It is projected to reach USD 1,056.5 million by 2035.
The market is expected to grow at a 6.0% CAGR between 2025 and 2035.
The key product types are plate agglutination mycoplasma plate antigen test products, mycoplasma plate antigens, mycoplasma plate antigens- control serum, plate antigens-negative control serum, confirmatory testing reagents, mycoplasma whole organism, mycoplasma antiserum and negative serum.
for drug development segment is expected to dominate with a 41.2% industry share in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Plate Chain Elevator Market Size and Share Forecast Outlook 2025 to 2035
Platelet Shaker Market Size and Share Forecast Outlook 2025 to 2035
Plate and Frame Heat Exchanger Market Size and Share Forecast Outlook 2025 to 2035
Platelet Concentration Systems Market Size and Share Forecast Outlook 2025 to 2035
Platelet Rich Plasma Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Plate Electrostatic Precipitator Market Size and Share Forecast Outlook 2025 to 2035
Platelet Function Test Market Report - Growth & Forecast 2025 to 2035
Plate Heat Exchanger Market Growth - Trends & Forecast 2025 to 2035
Plates Market
Tinplate Packaging Market Size and Share Forecast Outlook 2025 to 2035
Hotplate Stirrers Market Size and Share Forecast Outlook 2025 to 2035
Template Preparation Kits Market
Miniplate for Bone Fixation Market Size and Share Forecast Outlook 2025 to 2035
Drawplate Market Size and Share Forecast Outlook 2025 to 2035
PCR Plate Sealer Market Size and Share Forecast Outlook 2025 to 2035
Gold-plated Palladium Bonding Wire Market Size and Share Forecast Outlook 2025 to 2035
Cold Plates Market Size and Share Forecast Outlook 2025 to 2035
Microplate Handling Instruments Market Size and Share Forecast Outlook 2025 to 2035
Core Plate Varnishes Market Size and Share Forecast Outlook 2025 to 2035
Ship Plate Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA