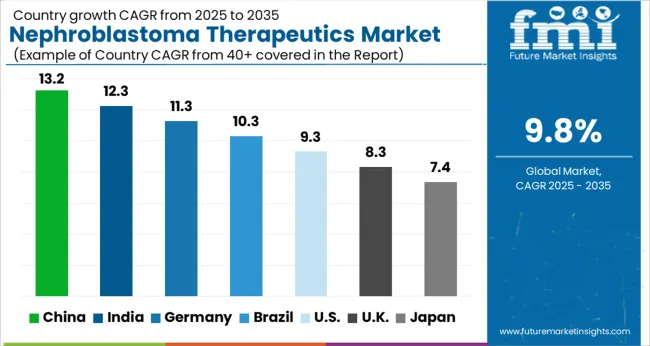
The Nephroblastoma Therapeutics Market is estimated to be valued at USD 2.6 billion in 2025 and is projected to reach USD 6.5 billion by 2035, registering a compound annual growth rate (CAGR) of 9.8% over the forecast period.
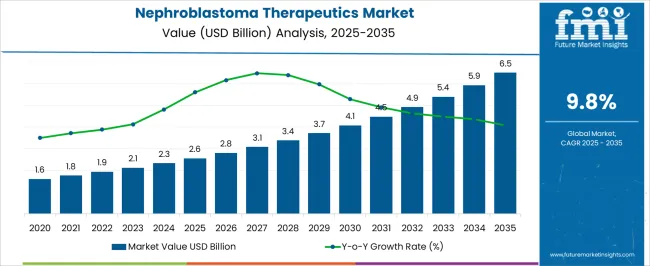
| Metric | Value |
|---|---|
| Nephroblastoma Therapeutics Market Estimated Value in (2025 E) | USD 2.6 billion |
| Nephroblastoma Therapeutics Market Forecast Value in (2035 F) | USD 6.5 billion |
| Forecast CAGR (2025 to 2035) | 9.8% |
The nephroblastoma therapeutics market is experiencing steady progress driven by advancements in oncology research, improved treatment protocols, and the integration of multidisciplinary approaches in pediatric oncology care. Growing emphasis on early diagnosis, rising survival rates through combined therapies, and expanding access to oncology drugs in emerging regions are shaping the market dynamics.
Pharmaceutical companies are channeling investments into developing more targeted and less toxic drug regimens to improve quality of life for young patients. Supportive regulatory frameworks and increasing collaborations between hospitals, research institutes, and cancer foundations are also facilitating accelerated availability of innovative treatments.
The market outlook remains positive as efforts continue to focus on reducing relapse rates, optimizing chemotherapy combinations, and expanding distribution networks to ensure accessibility of nephroblastoma therapies worldwide.
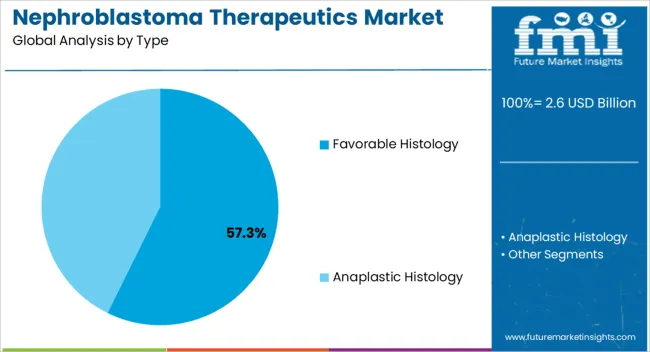
The favorable histology segment is projected to account for 57.30% of total market revenue by 2025 within the type category, making it the most prominent. This dominance is supported by the relatively better treatment outcomes and higher survival rates associated with favorable histology nephroblastoma compared to other subtypes.
The segment has benefited from standardized chemotherapy and surgical protocols that deliver consistent results, thereby reinforcing confidence among oncologists.
Continuous improvements in diagnostic imaging and pathology have also enhanced the identification and management of favorable histology cases, further supporting its leadership position.
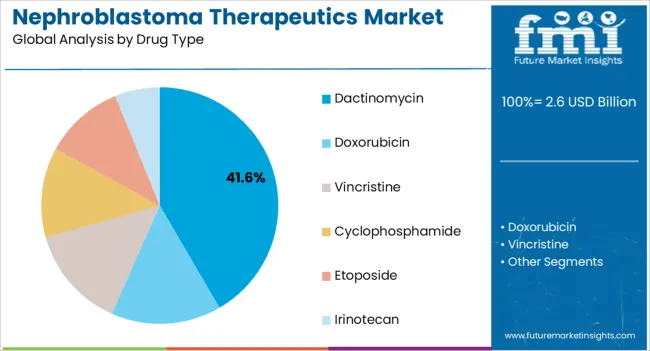
The dactinomycin segment is expected to contribute 41.60% of total revenue by 2025 within the drug type category, positioning it as the leading therapeutic option. Its widespread use in established treatment regimens for nephroblastoma, often in combination with other chemotherapeutic agents, has solidified its clinical importance.
The proven efficacy of dactinomycin in reducing tumor recurrence and improving long term survival outcomes has maintained its relevance in therapeutic protocols.
Additionally, its integration into global pediatric oncology guidelines has ensured consistent adoption across both developed and developing healthcare systems.
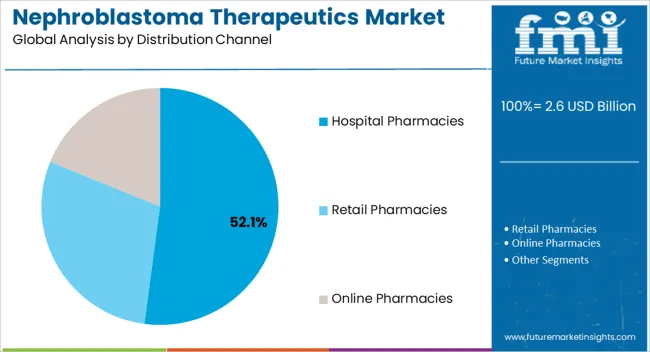
The hospital pharmacies segment is estimated to hold 52.10% of total revenue by 2025 within the distribution channel category, highlighting its dominance. This is primarily due to the critical nature of nephroblastoma treatment, which requires close supervision by oncology specialists and hospital based administration of chemotherapeutic agents.
Hospital pharmacies provide streamlined access to essential drugs, support adherence to treatment schedules, and ensure safe handling of potent oncology medications.
The segment’s prominence is further reinforced by the concentration of pediatric oncology expertise and supportive care infrastructure within hospital settings, making hospital pharmacies the central hub for nephroblastoma therapeutics distribution.
The global nephroblastoma therapeutics market grew at a CAGR of 8.88% from 2020 to 2025, as per Future Market Insights, a provider of market research and competitive intelligence.
Although most patients with a histological diagnosis of Wilms tumor do well with current treatment, approximately 10% of patients have histopathological features that are associated with a worse prognosis, and in some types, with a high incidence of relapse and death.
Nowadays, improved risk stratification has divided the population of patients into small subgroups, creating the challenge of designing and executing clinical trials that are sufficiently powered to demonstrate the desired outcomes. The fruits of stepped-up international collaboration are beginning to be realized, with the discovery of new genes, biological markers, and therapeutic targets. The benefits of COG-SIOP collaboration will hopefully translate into the application of evidence-based diagnostic and therapeutic approaches in low-income countries.
Increase in approvals of regulatory bodies
Key players in the market are focused on receiving approvals from regulatory bodies for drugs used to treat nephroblastoma is expected to drive the market growth over the forecast period.
Frequent regulatory approvals for new nephroblastoma treatments are expected to propel the growth of the global nephroblastoma treatment market during the forecast period. Market players are focusing on approvals from regulatory authorities for new drugs.
Focusing on the growth of R&D activities
Moreover, market players are focusing on R&D activities for new treatments, which is also expected to augment the growth of the global nephroblastoma treatment market during the forecast period. For instance, in August 2025, Children’s Oncology Group studying the combination of chemotherapy and surgery effects on young patients suffering from Wilms tumor revealed to be in Phase 3 trial and that the study is likely to be completed by end of the year 2025.
The high cost of treatment leads acts as a restraining factor
The major factor that hinders the growth of the global nephroblastoma therapeutics market includes the high cost of nephroblastoma. According to an article published by the American Society of Clinical Oncology, the cost for the full course of treatment was determined between USD 1490 and USD 2093 for a patient suffering from nephroblastoma.
Favorable reimbursement policies offering moneymaking opportunities in the United States
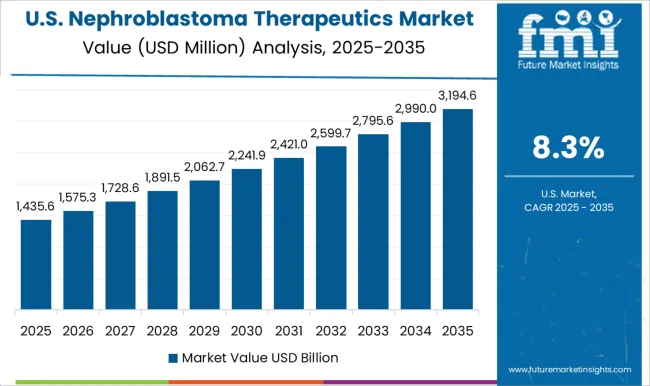
The United States is known for its healthcare infrastructure and favorable reimbursement scenario as both of them are expected to push the demand for nephroblastoma treatment shortly. A rise in the incidence of different types of tumors, rapid adoption of novel cancer treatments, and an increase in investment in medical research and development are also expected to favor market growth in the future.
Increasing investments in healthcare infrastructural development will favor market growth in Japan
Demand for nephroblastoma treatment is expected to be primarily driven by an increasing focus on healthcare infrastructure development and a rise in investments in medical R&D, the growing awareness regarding nephroblastoma, an increase in spending patterns on health and fitness, and the growing availability of novel cancer treatments with a rise in the number of cases are the factors that will augment the development of the market over the next ten years.
Dactinomycin is said to hold a dominant share of the market
According to the reports of FMI, of 13 children treated with dactinomycin in addition to nephrectomy and postoperative radiotherapy, 92% (12 of 13) are living and free of tumors. It was concluded that the survival of children with nephroblastoma is markedly prolonged by the administration of dactinomycin at the time of diagnosis. The beneficial effect of dactinomycin was attributed to the ‘cidal’ action of this drug on small or poorly established metastases.
The demand for hospital pharmacies will be more during the forecast period.
According to a report by Future Market Insights, the hospital pharmacies would grow within the anticipated time frame. This market sector is expected to hold a greater share of the worldwide market from 2025 to 2035.
Due to the growing number of people visiting hospitals for advice on treatment related to nephroblastoma, the hospital pharmacy is anticipated to dominate the worldwide nephroblastoma therapeutics market. As a result, individuals more pertain towards their health and well-being. Thus, the majority of consumers are expected to only buy medications from hospital pharmacies.
The Soonicorns - soon to be Unicorns - are the handful of highly valued startups that have successfully grown out of their nascency to attract valuations of over a few hundred million. Watch out for these companies in the coming years as they take on the journey to becoming elite Unicorns.
Penn Medicine’s Abramson Cancer Center published early data from the first USA clinical trial of CRISPR-edited immune cells, showing that genetically editing the T cells of human cancer patients and infusing the cells back into the patients appeared safe and feasible. Investigators reported infusing three participants - two with multiple myeloma and one with sarcoma.
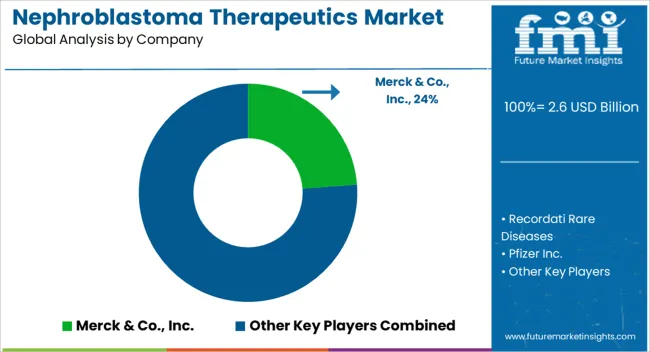
Some of the prominent players in the global market for Nephroblastoma Therapeutics Market treatment are
Some of the important developments of the key players in the market are
| Report Attributes | Details |
|---|---|
| Growth Rate | CAGR of 9.88% from 2025 to 2035 |
| Market value in 2025 | USD 2.6 billion |
| Market value in 2035 | USD 6.5 billion |
| Base Year for Estimation | 2025 |
| Historical Data | 2020 to 2025 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | USD Billion for Value and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends, and Pricing Analysis |
| Segments Covered | Type, Drug type, Distribution channel, Region |
| Regions Covered | North America; Latin America; Europe; South Asia; East Asia; Oceania; Middle East & Africa |
| Key Countries Profiled | United States, Canada, Brazil, Mexico, Rest of Latin America, Germany, United Kingdom, France, Spain, Italy, Rest of Europe, India, Malaysia, Singapore, Thailand, Rest of South Asia, China, Japan, South Korea, Australia, New Zealand, GCC countries, South Africa, Israel |
| Key Companies Profiled | Merck & Co., Inc.; Recordati Rare Diseases; Pfizer Inc.; Sun Pharmaceutical Industries Ltd.; Cipla Inc.; Actiza Pharmaceutical Private Limited; Teva Pharmaceutical Industries Ltd.; Alvogen; Accord Healthcare Ireland Ltd.; Amneal Pharmaceuticals LLC. |
| Customization Scope | Available on Request |
The global nephroblastoma therapeutics market is estimated to be valued at USD 2.6 billion in 2025.
The market size for the nephroblastoma therapeutics market is projected to reach USD 6.5 billion by 2035.
The nephroblastoma therapeutics market is expected to grow at a 9.8% CAGR between 2025 and 2035.
The key product types in nephroblastoma therapeutics market are favorable histology and anaplastic histology.
In terms of drug type, dactinomycin segment to command 41.6% share in the nephroblastoma therapeutics market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Biotherapeutics Virus Removal Filters Market Trends – Growth & Forecast 2025 to 2035
COPD Therapeutics Market Report – Growth, Demand & Industry Forecast 2023-2033
Digital Therapeutics and Wellness Market Size and Share Forecast Outlook 2025 to 2035
Digital Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Peptide Therapeutics Market Analysis - Growth & Forecast 2024 to 2034
Advanced Therapeutics Pharmaceutical Outsourcing Market Size and Share Forecast Outlook 2025 to 2035
Glaucoma Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Leukemia Therapeutics Treatment Market Analysis - Growth & Forecast 2025 to 2035
Microbiome Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
The Canine Flu Therapeutics Market is segmented by product, and end user from 2025 to 2035
Stuttering Therapeutics Market Trends, Analysis & Forecast by Treatment, Type, End-Use and Region through 2035
Pet Cancer Therapeutics Market Insights - Growth & Forecast 2024 to 2034
Candidiasis Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Lung Cancer Therapeutics Market Analysis – Size, Share, and Forecast Outlook 2025 to 2035
Heart Block Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Aquaculture Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Fucosidosis Therapeutics Market - Growth & Innovations 2025 to 2035
Market Leaders & Share in Alzheimer’s Therapeutics
Alzheimer’s Therapeutics Market Analysis by Disease Class into Cholinesterase Inhibitors, NMDA Receptor Antagonists and Combinations Through 2035.
Sarcoidosis Therapeutics Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA