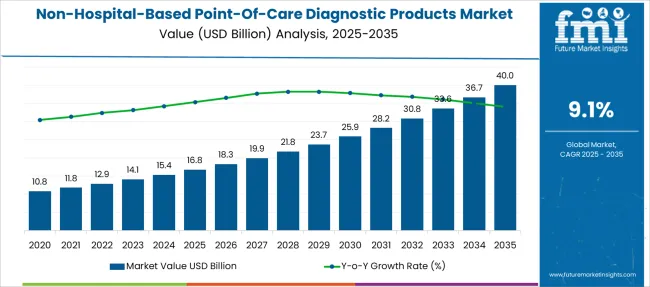
The Non-Hospital-Based Point-Of-Care Diagnostic Products Market is estimated to be valued at USD 16.8 billion in 2025 and is projected to reach USD 40.0 billion by 2035, registering a compound annual growth rate (CAGR) of 9.1% over the forecast period.
| Metric | Value |
|---|---|
| Non-Hospital-Based Point-Of-Care Diagnostic Products Market Estimated Value in (2025 E) | USD 16.8 billion |
| Non-Hospital-Based Point-Of-Care Diagnostic Products Market Forecast Value in (2035 F) | USD 40.0 billion |
| Forecast CAGR (2025 to 2035) | 9.1% |
The non-hospital-based point-of-care diagnostic products market is expanding rapidly, driven by increased demand for convenient and rapid testing solutions outside traditional healthcare facilities. Growing consumer awareness about health monitoring and the need for quick diagnosis have led to widespread adoption of point-of-care devices.
The shift toward decentralized healthcare and the rising prevalence of chronic diseases have increased reliance on homecare testing products. Advances in product design, especially the development of user-friendly and affordable diagnostic kits, have made self-testing accessible to a broad population.
Health agencies and community health initiatives have further encouraged the use of these products to reduce hospital visits and lower healthcare costs. The market outlook remains positive with ongoing innovation in diagnostic accuracy and the expansion of testing applications. Segmental growth is expected to be driven by paper-based test kits for their simplicity and cost-effectiveness, along with homecare settings as the primary end users due to increasing demand for at-home health monitoring.
The market is segmented by Product Type and End User and region. By Product Type, the market is divided into Paper to based Test Kit and Analyzer. In terms of End User, the market is classified into Homecare Settings, Diagnostic Clinics, Clinics, and Others. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
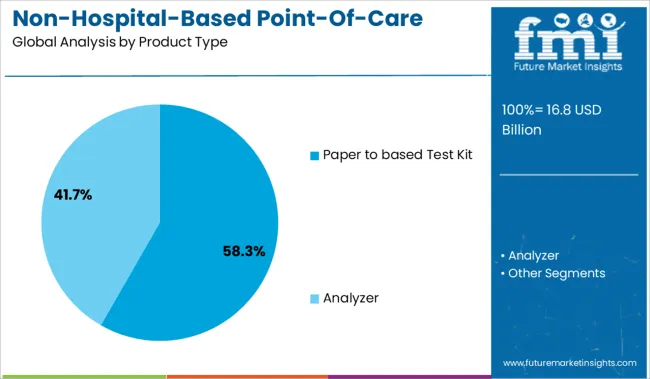
The paper-based test kit segment is projected to hold 58.3% of the market revenue in 2025, establishing it as the leading product type. This growth is attributed to the affordability and ease of use of paper-based kits, which require minimal training and no specialized equipment. Their portability and rapid result delivery make them ideal for use in remote or resource-limited settings.
The simple design reduces manufacturing costs, enabling mass production and wide distribution. Furthermore, these kits are favored for screening common conditions and infections, supporting early diagnosis and timely intervention.
The disposability and environmentally friendly nature of paper-based kits align with increasing consumer preference for sustainable healthcare products. These factors contribute to the dominant market position of the paper-based test kit segment.
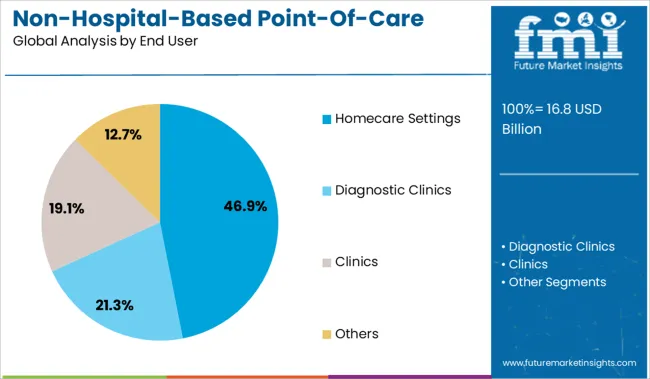
The homecare settings segment is expected to contribute 46.9% of the market revenue in 2025, maintaining its lead as the primary end user. The growth in this segment is driven by the convenience of conducting diagnostic tests at home, especially among aging populations and those with chronic illnesses requiring regular monitoring.
Homecare testing reduces dependency on hospital visits and facilitates early detection and management of health conditions. Healthcare providers increasingly recommend home testing as part of patient-centric care models.
The expansion of telehealth services has also complemented the use of home-based diagnostic products by enabling remote consultation and follow-up. Rising consumer confidence in self-testing and ongoing improvements in test accuracy support the continued expansion of the homecare settings segment.
The increasing geriatric population, rising incidence of diabetes, and unmet need for home care are growing the non-hospital-based point-of-care diagnostic products market trends in recent years.
The growing prevalence of target diseases coupled with an increase in the geriatric population base, which is more prone to disease development and needs home care and monitoring, is anticipated to rise in the non-hospital-based point-of-care diagnostic products market future trends over the forecast period.
Technology advancement, cost-effective products, and high accuracy are the factors that are anticipated to grow the non-hospital-based point-of-care diagnostic products market key trends & forecast in the coming year. Moreover, the availability of advanced rapid diagnostic tests and increasing awareness have also boosted the growth of non-hospital-based point-of-care diagnostic products market analysis over the forecast period.
However, improper care of equipment, lack of quality control, and poor training can hamper the global non-hospital-based point-of-care diagnostic products market growth over the forecast period from 2025 to 2035.
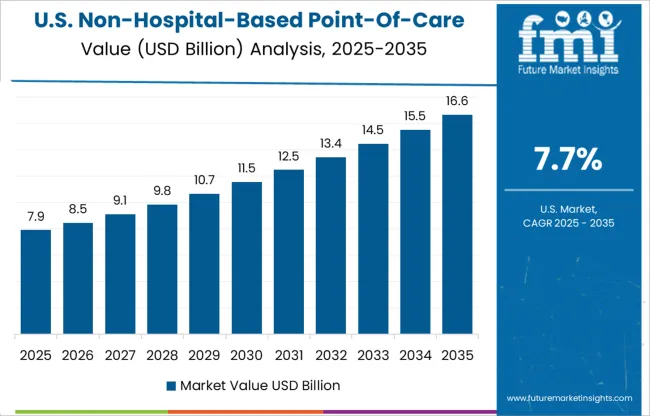
By region, the global non-hospital-based point-of-care diagnostic products market share is anticipated to dominate the North American region during the forecast period from 2025 to 2035.
North America acquires 33.2% of the share in the non-hospital-based point-of-care diagnostic products market share all around the region. Due to the high incidence of metabolic disease moreover, favourable government regulations and initiatives for the development of healthcare infrastructure are expected to result in higher demand for Non-hospital-based point-of-care diagnostic products over the forecast period.
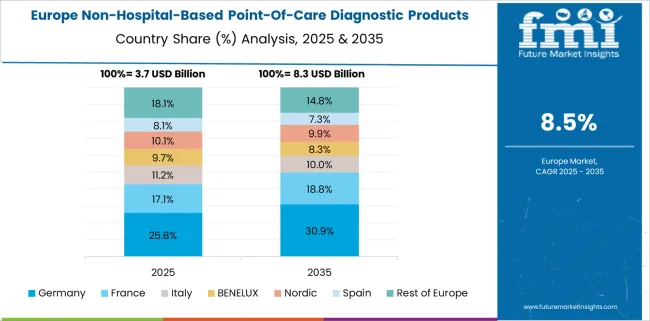
The Europe region is expected to be the most attractive region that stood in second place in the non-hospital-based point-of-care diagnostic products market size acquiring 29.8% of the share during the forecast period from 2025 to 2035.
Due to owing to the presence of high unmet medical needs and constantly improving healthcare infrastructure, growing medical awareness, and a rise in per capita income are likely to grow the non-hospital-based point-of-care diagnostic products market key trends & opportunities in the region.
Recently, the non-hospital-based point-of-care diagnostic products market trends are growing & focusing on consumers' needs, cost-saving supply chains, and automation which are anticipated to acquire a significant non-hospital-based point-of-care diagnostic products market growth in recent years.
The non-hospital-based point-of-care diagnostic product statistics are developing in microfluidic, assay formats, biosensors, bioanalytical platforms, and other complementary techniques during the forecast period from 2025 to 2035.
A biosensor is one of the effective components of non-hospital-based point-of-care diagnostic products, which directly affects the bioanalytical performance. Some of the biosensors are surface plasmon and white light reflectance spectroscopy, and others are likely to develop to improve the non-hospital-based point-of-care diagnostic products market share.
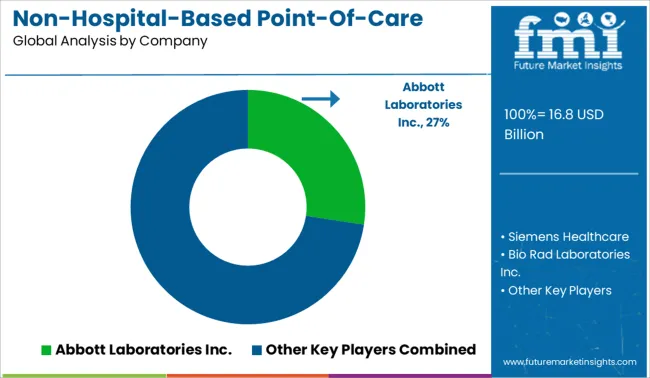
The key players in the Non-hospital-based point-of-care diagnostic products market are Abbott Laboratories Inc., Siemens Healthcare GmbH, Bio Rad Laboratories Inc., Thermo Fisher Scientific Inc., and Roche Diagnostics (H. Hoffman-La Roche Ltd.), STD Rapid Test Kits, etc.
Most of the companies sell their products through medical equipment distributors that operate in different regions and the distribution network and market presence are likely to grow the sales of non-hospital-based point-of-care diagnostic products in recent years.
Recent Development in the Non-Hospital-Based Point-Of-Care diagnostic Products Market are:
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 9.1% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in USD Million and CAGR from 2025 to 2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered | Product Type, End User, Region |
| Regions Covered | North America; Latin America; Europe; East Asia; South Asia; Oceania; Middle East and Africa |
| Key Countries Profiled | USA, Canada, Brazil, Argentina, Germany, UK, France, Spain, Italy, Nordics, BENELUX, Australia & New Zealand, China, India, ASEAN, GCC, South Africa |
| Key Companies Profiled | Abbott Laboratories Inc.; Siemens Healthcare GmbH; Bio Rad Laboratories Inc.; Thermo Fisher Scientific Inc.,; Roche Diagnostics (H. Hoffman-La Roche ;Ltd.); STD Rapid Test Kits |
| Customization | Available Upon Request |
The global non-hospital-based point-of-care diagnostic products market is estimated to be valued at USD 16.8 billion in 2025.
The market size for the non-hospital-based point-of-care diagnostic products market is projected to reach USD 40.0 billion by 2035.
The non-hospital-based point-of-care diagnostic products market is expected to grow at a 9.1% CAGR between 2025 and 2035.
The key product types in non-hospital-based point-of-care diagnostic products market are paper to based test kit, _pregnancy test kit, _infection test kit, _glucose test kit, _others, analyzer, _glucose monitor, _pulse oximetry and _others.
In terms of end user, homecare settings segment to command 46.9% share in the non-hospital-based point-of-care diagnostic products market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Point-of-Care Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Point-of-care Molecular Diagnostics Market Insights – Trends & Growth 2025 to 2035
Products from Food Waste Industry Analysis in Korea Size, Share and Forecast Outlook 2025 to 2035
Products from Food Waste in Japan - Size, Share, and Forecast Outlook 2025 to 2035
Products from Food Waste Market Analysis - Size, Growth, and Forecast 2025 to 2035
USA Products from Food Waste Market Growth – Trends, Demand & Outlook 2025-2035
Teff Products Market
Detox Products Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Algae Products Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Pulse Products Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Dairy Products Market Analysis by Product Type, End Use, Distribution Channel and Region through 2035
Almond Products Market Size and Share Forecast Outlook 2025 to 2035
Bamboo Products Market Analysis – Trends & Growth 2025 to 2035
Luxury Products For Kids Market - Trends, Growth & Forecast 2025 to 2035
Chicory Products Market Size and Share Forecast Outlook 2025 to 2035
Crystal Products Market Size and Share Forecast Outlook 2025 to 2035
Make-Up Products Packaging Market Size and Share Forecast Outlook 2025 to 2035
Suncare Products Market Size and Share Forecast Outlook 2025 to 2035
Ziplock Products Market Size and Share Forecast Outlook 2025 to 2035
Global Moringa Products Market Outlook – Trends, Demand & Forecast 2025–2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA