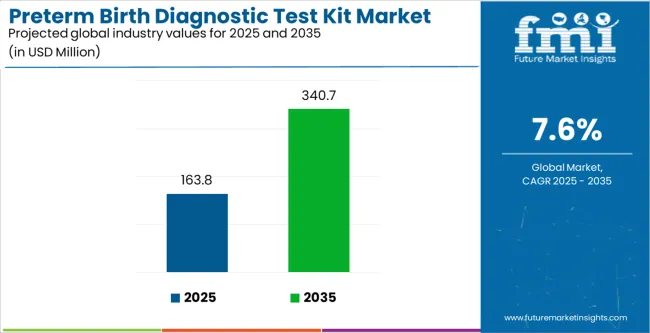
The preterm birth diagnostic test kit market is projected to grow from USD 163.8 million in 2025 to USD 340.7 million by 2035, at a CAGR of 7.6% during the forecast period. Market dynamics are influenced by growing global concern regarding preterm births, which remain a leading cause of neonatal morbidity and mortality. Healthcare systems are increasingly prioritizing early detection and preventive care, driving higher adoption of advanced diagnostic kits. Rising awareness among both healthcare providers and expectant mothers is fueling demand for reliable, rapid, and non-invasive diagnostic solutions.
A major driver shaping this market is the integration of molecular diagnostic methods, including biomarkers such as fetal fibronectin and cervical length assessments, which improve prediction accuracy. The push toward point-of-care testing is another key factor, as it enables clinicians to make quick, evidence-based decisions in emergency and community settings. Expanding healthcare infrastructure in emerging economies is also opening access to preterm birth screening, particularly where maternal health challenges are more acute.
On the opportunity side, innovation in portable diagnostic devices and lab-on-chip platforms is expected to expand the scope of testing in both hospital and non-hospital environments. Strategic partnerships between diagnostic kit manufacturers, maternal health organizations, and governments are fostering wider adoption through public health programs. The rising use of AI-powered diagnostic tools for risk assessment is also likely to enhance predictive capabilities, reducing false positives and enabling targeted intervention strategies.
Challenges remain, particularly the high cost of advanced diagnostic kits in low- and middle-income countries, which limits affordability and widespread adoption. Variability in regulatory frameworks across regions also creates barriers to faster commercialization. However, ongoing efforts to standardize guidelines for preterm birth testing and growing international collaboration are gradually easing these constraints.
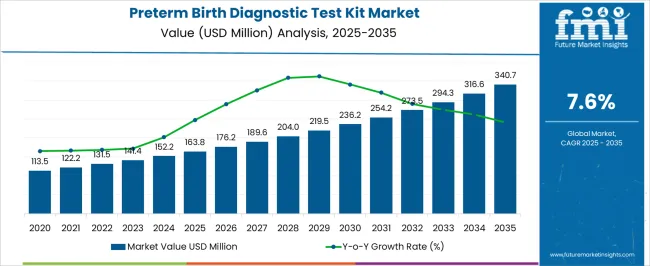
| Metric | Value |
|---|---|
| Preterm Birth Diagnostic Test Kit Market Estimated Value in (2025 E) | USD 163.8 million |
| Preterm Birth Diagnostic Test Kit Market Forecast Value in (2035 F) | USD 340.7 million |
| Forecast CAGR (2025 to 2035) | 7.6% |
The market is segmented by Product, Sample, and End User and region. By Product, the market is divided into PAMG-1 Test, FFN Test, and IGFBP-1 Test. In terms of Sample, the market is classified into Blood, Urine, and Vaginal Discharge. Based on End User, the market is segmented into Hospitals, Diagnostic Laboratories, Outpatient Clinics, and Research Centres. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
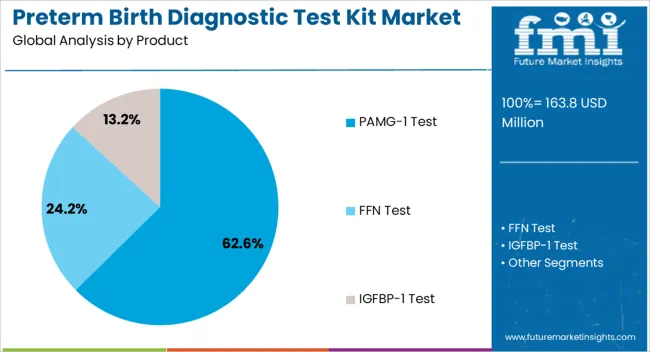
The PAMG-1 Test segment is projected to account for 62.6% of the preterm birth diagnostic test kit market revenue in 2025, establishing itself as the leading product type. This segment’s prominence has been driven by PAMG-1’s superior sensitivity and negative predictive value in detecting imminent spontaneous preterm delivery.
Peer-reviewed clinical studies and obstetric guidelines have consistently recommended PAMG-1 tests for their reliability in managing symptomatic women presenting with preterm labor signs. Hospitals and maternity centers have increasingly adopted PAMG-1 testing due to its rapid result turnaround and low false-positive rates, which aid in reducing unnecessary hospitalizations and interventions.
Diagnostic companies have focused on expanding the availability of PAMG-1 tests through both laboratory-based and point-of-care platforms, improving access in a variety of clinical settings. As clinical validation continues to support its diagnostic efficacy, the PAMG-1 Test segment is expected to maintain its leadership position in preterm birth diagnostics.
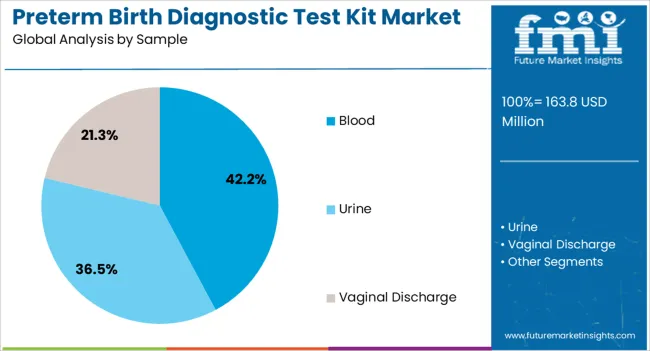
The Blood segment is projected to capture 42.2% of the preterm birth diagnostic test kit market revenue in 2025, reflecting its growing adoption as a preferred sample type. This growth has been attributed to the minimally invasive nature of blood collection, which enhances patient comfort and simplifies integration into prenatal care workflows.
Diagnostic test developers have increasingly focused on blood-based biomarker assays due to their compatibility with standard laboratory equipment and high reproducibility. Hospitals and reference labs have favored blood samples for their ease of transport, storage, and automation potential.
Furthermore, clinical research has identified several blood biomarkers with strong correlation to preterm birth risk, encouraging broader test development. With improvements in detection sensitivity and ongoing clinical trials validating newer panels, the Blood segment is expected to maintain steady market growth, supported by its operational efficiency and clinical acceptance.
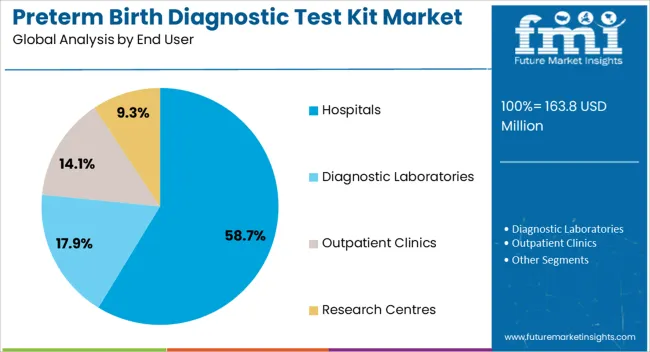
The Hospitals segment is projected to contribute 58.7% of the preterm birth diagnostic test kit market revenue in 2025, maintaining its lead among end users. This segment’s growth has been fueled by the central role of hospitals in managing high-risk pregnancies and providing immediate care in cases of preterm labor.
Obstetrics departments within hospitals have increasingly implemented standardized protocols that include diagnostic testing for early identification of preterm birth risk, especially among symptomatic patients. Institutional purchasing power, access to diagnostic infrastructure, and integration with electronic medical records have facilitated test adoption across hospital networks.
In addition, hospitals have served as primary sites for clinical research, aiding in the validation and deployment of advanced diagnostic kits. Public health programs and maternal health initiatives have also prioritized hospital-based screening to improve perinatal outcomes. As hospitals continue to serve as the front line for comprehensive maternal and neonatal care, this segment is expected to remain dominant in the deployment of preterm birth diagnostic test kits.
Growth Determinants in the Preterm Birth Screening Kit Industry
Childbirth before 37 weeks of pregnancy, termed preterm birth, is a noteworthy health issue globally. The greater frequency of preterm births amplifies the demand for precise and steady preterm birth diagnostic screening kits to recognize and speculate preterm labor.
This catalyst has the potential to continue as medical care providers and governments emphasize curbing preterm birth rates and enhancing maternal and newborn health results. This factor strengthens the global growth of the preterm birth diagnostic test kit market.
Progress in medical technology has led to the innovations of experienced and precise diagnostic test kits for envisioning preterm birth. The preterm birth diagnostic test kits adopt techniques like genetic testing, biomarker analysis, and ultrasound estimations. As this equipment develops and is widely accessible, it aids the growth of the preterm birth diagnostic test kit industry.
Deterrents Impeding the Preterm Birth Diagnostic Testing Equipment Sector
The medical diagnostic sector is administered to assure patient security and product efficiency. Making and introducing new diagnostic test kits to the industry needs intense testing and approval procedures. Regulatory obstacles hinder the launch of new test kits to the sector, adversely influencing the adoption of preterm birth diagnostic test kits.
Few advanced diagnostic test kits are expensive to research, manufacture, and buy. The cost stimulus constrains availability, especially in economies with fewer healthcare resources or where medical care is not well-resourced to dispense modern diagnostic services. Towering costs inhibit healthcare amenities from embracing new technologies, impeding preterm birth diagnostic test kit market growth.
Between 2020 and 2025, the preterm birth diagnostic test kit market saw steady growth because of booming awareness regarding preterm birth intricacies and diagnostic technology innovations.
The preterm birth diagnostic testing kit sector evolved at a CAGR of 7.2% from 2020 to 2025, catapulted by the soaring prevalence of preterm births and schemes by healthcare institutions to constrain neonatal casualty rates. Partnerships between healthcare organizations and diagnostic kit producers amplified the preterm birth diagnostic testing equipment market expansion.
From 2025 to 2035, the preterm birth diagnostic test kit demand is likely to strengthen significantly. This escalation is characterized by numerous factors, like an aging population, rising maternal age, lifestyle shifts resulting in higher occurrences of preterm births, and the launch of modern diagnostic solutions.
The government schemes targeted at enhancing maternal and child healthcare in developing economies flourish the growth of the preterm birth diagnostic test kit sector.
The evolution in precision medicine and tailored healthcare soars the adoption of progressive diagnostic techniques, intensifying the preterm birth diagnostic test equipment adoption. The preterm birth diagnostic test kit manufacturers stress product innovation and partnerships to take advantage of emerging prospects and cater to shifting customer requirements.
The section below covers the forecast for the preterm birth diagnostic test equipment market in terms of countries. Information on key countries in several parts of the globe, including North America, Asia Pacific, Europe, and others, is provided.
The United States remains at the forefront in North America, with a CAGR of 8.1% through 2035. In the Asia Pacific region, China is projected to witness a CAGR of 10.3% by 2035, leaving behind India at 8.6%.
| Countries | CAGR (From 2025 to 2035) |
|---|---|
| United States | 8.1% |
| Germany | 7.3% |
| France | 7.5% |
| China | 10.3% |
| India | 8.6% |
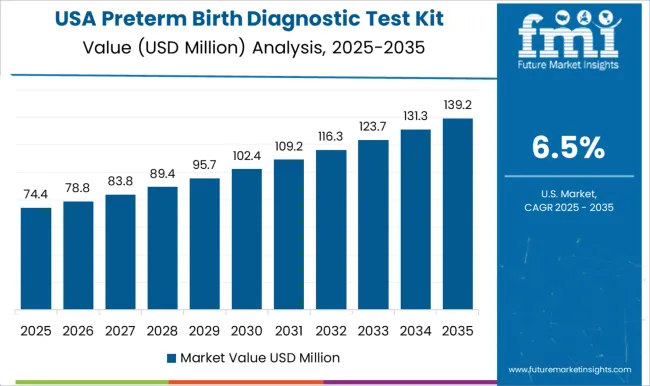
Technological innovations and strong research escalated the advancement and adoption of preterm birth diagnostic tests in the United States. The United States exhibits modern healthcare infrastructure, prospectively intensifying the demand for preterm birth diagnostic test kits.
The occurrence of risk factors like obesity and maternal age highlights the requirement for productive preterm birth screening techniques in the United States
With a focus on prenatal care, there is a responsive sector for dependable preterm birth diagnostic kits in the United States. Strong government schemes and medical care policies stimulate the sales of preterm birth diagnostic testing equipment in the United States.
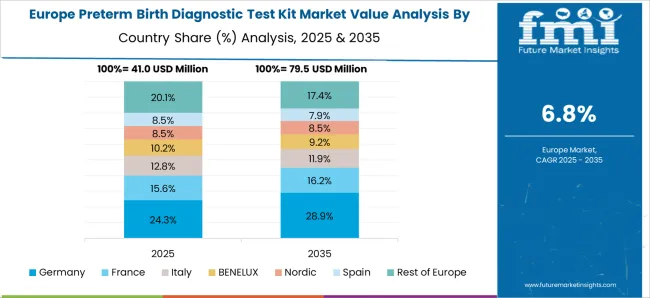
The recognized healthcare system in Germany stimulates the broad adoption of preterm birth diagnostic kits. With a focus on preventive healthcare, the rising interest in early screening kits for preterm birth in German healthcare providers is a documentation of their commitment and significance in transforming the healthcare future.
Considerable attention to child and maternal health initiatives presents opportunities for combining preterm birth diagnostic solutions into the traditional healthcare ecosystem in Germany. The aging population and falling birth rates in Germany accentuate the necessity of precise preterm birth tests in maternal health care.
The comprehensive healthcare coverage in France facilitates the adoption of preterm birth diagnostic test kits. A culturally ingrained focus on maternal and child health intensifies the demand for preterm birth screening kits in France. French government schemes to minimize preterm birth rates encourage investments in diagnostic technologies customized to the healthcare ecosystem.
The presence of medical research and innovation in France prompts the innovation of modern preterm birth diagnostic solutions. The surging consciousness among pregnant women and medical care providers about the concerns of preterm birth strengthens the demand for diagnostic alternatives in France.
The section contains information about the leading sectors in the preterm birth diagnostic testing equipment industry. In terms of product category, the PAMG-1 Test segment is estimated to account for a share of 62.6% by 2035. By sample type category, the blood segment is projected to dominate by holding a share of 42.2% in 2035.
| Segment | PAMG-1 Test |
|---|---|
| Value Share (2035) | 62.6% |
The PAMG-1 Test is efficient in identifying preterm labor risks and provides timely intervention, curbing the chances of complications. Healthcare providers look out for PAMG-1 because of its proven dependability and ease of application in medical settings. PAMG-1 has a proven track record that highlights its role in augmenting maternal and neonatal results.
The choice for PAMG-1 emerges because of its proficiency in serving the essential demand for preterm birth projection, boosting its sales. The ascendancy of PAMG-1 indicates the recognition of its key role in strengthening preterm birth management techniques.
| Segment | Blood |
|---|---|
| Value Share (2035) | 42.2% |
The recognized infrastructure for blood sample collection and diagnosis assists the demand for blood-based diagnostic kits. Healthcare providers value the exhaustive insights presented by blood tests, sustaining knowledgeable decision-making and customized patient care.
The ongoing innovations in blood-based diagnostic technologies improve the efficiency of preterm birth diagnostic test kits, and escalate the market growth.
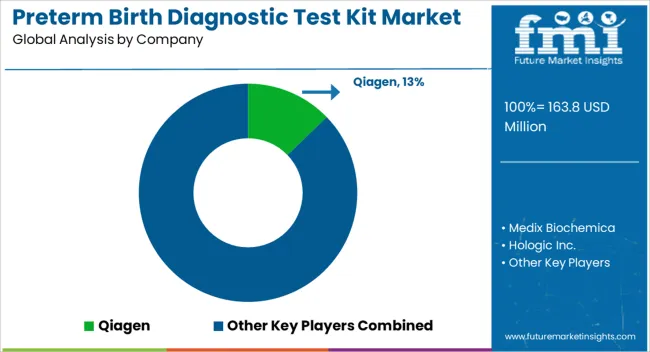
The market characterizes a myriad landscape with preterm birth diagnostic testing equipment vendors contending for a significant share. The leading manufacturers use technological innovations and medical research to present groundbreaking solutions.
The reputed preterm birth diagnostic test kit producers, along with rising startups, said the market dynamics, escalating competition through differentiation, strategies, and regional expansion.
Regulatory authorizations and collaborations with healthcare centers are critical for market entry. The continuous research and partnerships target improving diagnostic precision and expanding market access. The competitive landscape exhibits a blend of recognized preterm birth diagnostic testing kit vendors and startups vying to cater to persistent healthcare requirements linked to preterm birth.
Industry Updates
| Company | Sera Prognostics |
|---|---|
| Headquarter | United States |
| Recent Advancement |
|
| Company | Nuvo Group |
|---|---|
| Headquarter | Israel |
| Recent Advancement | Nuvo Group got the United States FDA approval for the expanded application of its INVU, a prescription-based remote pregnancy monitoring system to add a uterine activity module that allows remote screening of uterine activity. |
The report consists of key products like ffN test, PAMG-1 test, and IGFBP-1 test.
The market is trifurcated into blood, urine, and vaginal discharge.
Key end users present in the industry include hospitals, diagnostic laboratories, outpatient clinics, and research centers.
Analysis of the market has been carried out in key countries of North America, Latin America, East Asia, South Asia and Pacific, Western Europe, Eastern Europe, and the Middle East and Africa.
The global preterm birth diagnostic test kit market is estimated to be valued at USD 163.8 million in 2025.
The market size for the preterm birth diagnostic test kit market is projected to reach USD 340.7 million by 2035.
The preterm birth diagnostic test kit market is expected to grow at a 7.6% CAGR between 2025 and 2035.
The key product types in preterm birth diagnostic test kit market are pamg-1 test, ffn test and igfbp-1 test.
In terms of sample, blood segment to command 42.2% share in the preterm birth diagnostic test kit market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Preterm Birth Prevention and Management Market Analysis - Size, Share, and Forecast 2025 to 2035
Preterm Births and PROM Testing Market Size and Share Forecast Outlook 2025 to 2035
Birth Tissue Products Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Doula & Birth Coaching Services Market Trends – Growth to 2035
Kitchen/ Toilet Roll Converting Machines Market Size and Share Forecast Outlook 2025 to 2035
Kitting Robots Market Size and Share Forecast Outlook 2025 to 2035
Kitchen Tools and Accessories Market Size and Share Forecast Outlook 2025 to 2035
Kitchen Trailers Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Kitchen Hood System Market Size and Share Forecast Outlook 2025 to 2035
Kitchen Hand Tools Market Size and Share Forecast Outlook 2025 to 2035
Kitchen Hood Market Insights – Trends & Growth Forecast 2025 to 2035
Kitchen Islands and Carts Market
Kitchen Storage Market
Kitchen & Dining Furniture Market
Kitten Milk Replacer & Formula Market
Hip Kits Market Size and Share Forecast Outlook 2025 to 2035
Toy Kitchens and Play Food Market Size and Share Forecast Outlook 2025 to 2035
MEA Kitchen Storage Market Growth – Trends & Forecast 2025 to 2035
Meal Kit Market Size and Share Forecast Outlook 2025 to 2035
Meal Kit Delivery Service Market - Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA