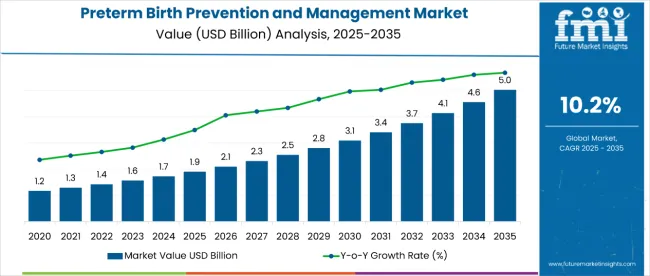
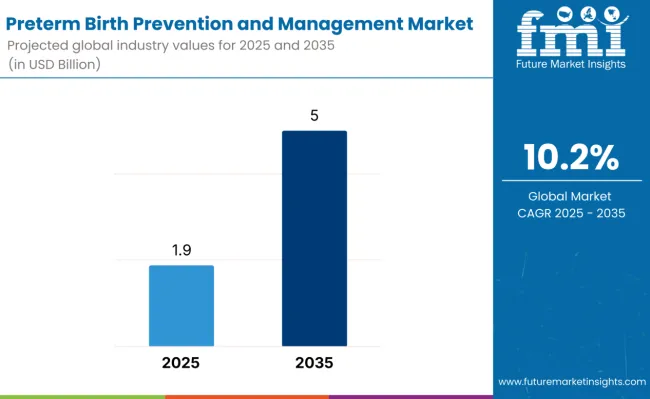
The preterm birth prevention and management market is estimated to be valued at USD 1.9 billion in 2025 and is projected to reach USD 5 billion by 2035, registering a compound annual growth rate (CAGR) of 10.2% over the forecast period.
| Metric | Value |
|---|---|
| Estimated Size (2025E) | USD 1.9 billion |
| Projected Value (2035F) | USD 5 billion |
| CAGR (2025 to 2035) | 10.2% |
The market is projected to increase by USD 3.1 billion over the forecast period, reflecting a total growth of 2.63 times from 2025 to 2035, at a CAGR of 10.2%. The market evolution is expected to be shaped by rising preterm birth incidences, increased adoption of progesterone therapy, and expanded use of parenteral administration routes across both emerging and developed healthcare markets.
By 2030, the market is likely to reach approximately USD 3.2 billion, with USD 1.3 billion added during the first half of the decade and USD 1.9 billion during the second half, reflecting back-loaded growth driven by expanding access to maternal healthcare and increasing awareness of preterm birth prevention therapies.
Companies such as Teva Pharmaceuticals USA, Inc., Pfizer Inc., Novartis AG, and Sanofi S.A. are advancing their competitive positions through investment in R&D for novel progesterone formulations and scalable parenteral delivery systems. Strategic collaborations and regional expansion are supporting penetration into emerging markets. Market performance will remain anchored in clinical efficacy, regulatory approvals, and growing adoption of standardized preterm birth prevention protocols across hospitals and specialty clinics.
The market holds a strategic position across its parent healthcare sectors, reflecting its increasing adoption in maternal and neonatal care. Within the global maternal health market, preterm birth prevention therapies contribute approximately 12% of the total, driven by progesterone adoption. In the women’s health pharmaceutical segment, it holds about 8%, highlighting its role in specialized therapeutic care.
Within reproductive medicine, its share is around 15%, supported by preventive applications. In the parenteral drugs market, it accounts for 5%, owing to injectable formulations. Collectively, these shares underscore its critical importance in hospital-based maternal care and pharmaceutical applications.
The market is driven by rising preterm birth rates, increased awareness of maternal and neonatal health, and expanding adoption of progesterone therapy. Innovations include sustained-release and injectable formulations that enhance compliance and clinical outcomes. Developments involve early screening programs, updated clinical guidelines, and regional capacity expansion.
Trends indicate increased integration of digital maternal health monitoring, personalized treatment protocols, and collaborations between pharmaceutical companies and healthcare providers to improve preventive care delivery. Regulatory compliance and clinical efficacy continue to guide market growth and competitive positioning.
Preterm birth prevention therapies’ unique ability to reduce the risk of premature delivery, improve neonatal outcomes, and support maternal health is driving their adoption. Their functional properties make them indispensable in managing high-risk pregnancies, where efficacy, safety, and timely intervention are critical for prolonging gestation and ensuring healthy newborn development.
Investments in maternal healthcare infrastructure, advanced treatment protocols, and availability of parenteral and oral therapies are fueling market growth. Healthcare providers prioritize therapies that improve gestational age, reduce neonatal complications, and offer cost-effective, evidence-based interventions.
Government initiatives, supportive insurance coverage, and rising accessibility to hospital and retail pharmacy distribution channels are strengthening the market outlook. With research focusing on improved formulations, safety, and patient-specific treatments, the market is expected to witness strong adoption across developed and emerging regions, particularly in countries with high preterm birth rates.
The market is segmented by therapy type, route of administration, patient type, distribution channel, and region. By therapy type, the market is divided into progesterone therapy, corticosteroid therapy, tocolytics therapy, antihypertensive therapy, magnesium sulfate therapy, heparin prophylaxis therapy, low-dose aspirin therapy, and antibiotics therapy.
Based on route of administration, the market is segmented into oral, parenteral, and vaginal. In terms of patient type, the market is categorized into prior spontaneous preterm birth, preeclampsia, short cervix, chronic hypertension, insulin-dependent, twins, antiphospholipid antibody syndrome, and others.
By distribution channel, the market is classified into hospital pharmacies, retail pharmacies, drug stores, and mail-order pharmacies. Regionally, the market spans North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, and the Middle East & Africa.
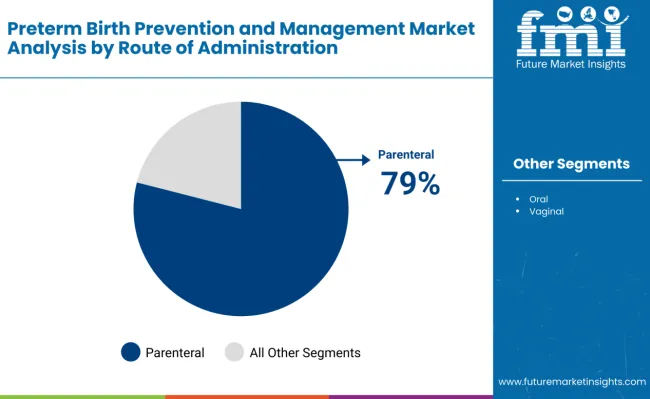
Parenteral administration dominates the route category with 79% market share, driven by rapid action, reliable bioavailability, and suitability for high-risk pregnancies. Injectable formulations of progesterone and other drugs ensure consistent therapeutic doses, making them the preferred choice in hospitals and maternal care centers.
Healthcare providers favor parenteral delivery because it allows precise dosing, improves patient compliance, and integrates seamlessly into both inpatient and outpatient maternal care protocols. It has become essential for clinicians managing pregnancies with elevated risk of premature labor.
Ongoing improvements in needle technology, formulation stability, and patient-friendly delivery systems are enhancing safety and effectiveness. With increasing adoption of clinical guidelines, expanding hospital infrastructure, and rising maternal health awareness globally, the parenteral segment is expected to maintain its leadership throughout the forecast period.
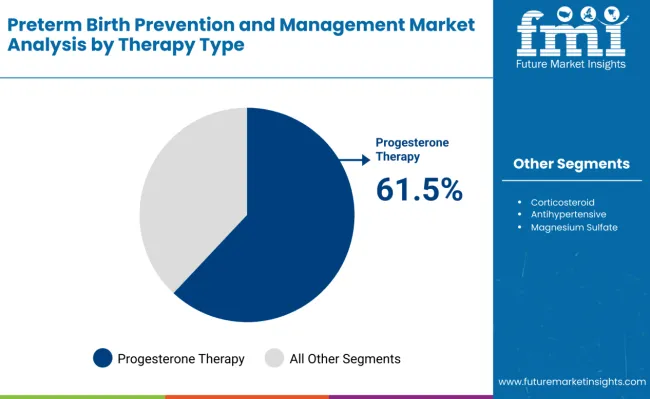
The progesterone therapy segment holds a dominant position with 61.5% of the market share in the therapy type category, driven by its proven effectiveness in preventing preterm births among high-risk pregnancies. Progesterone therapy is extensively used for women with prior spontaneous preterm birth, short cervix, or other maternal risk factors, providing consistent clinical outcomes and reducing neonatal complications.
Healthcare providers prefer progesterone therapy because it allows targeted preventive care, ensures patient compliance, and integrates seamlessly into hospital protocols. It has become indispensable for obstetricians and maternal care centers aiming to improve birth outcomes and minimize neonatal intensive care requirements.
Ongoing investments in sustained-release formulations, injectable delivery systems, and clinical research are enhancing efficacy, safety, and patient convenience. As awareness of maternal health grows globally, the progesterone therapy segment is expected to retain its leadership by enabling standardized preventive care for preterm birth.
In 2024, the global preterm birth prevention and management market expanded significantly, with North America accounting for a substantial share due to advanced maternal healthcare infrastructure. Key applications include progesterone therapy, corticosteroids, tocolytics, and antihypertensive interventions aimed at reducing preterm birth rates and improving neonatal outcomes. Healthcare providers and hospitals are increasingly adopting parenteral and sustained-release formulations to improve efficacy and patient compliance. Rising awareness of maternal and neonatal health, government initiatives, and investments in maternal care programs are strengthening market adoption.
Rising Maternal Healthcare Demand Drives Preterm Birth Prevention Adoption
The increasing prevalence of preterm births and growing focus on maternal and neonatal health are driving the adoption of preterm birth prevention and management therapies. Progesterone therapy, corticosteroids, and tocolytics are widely used in hospitals and clinics to reduce premature delivery risks and improve neonatal outcomes.
Expanding healthcare infrastructure, rising awareness among expectant mothers, and increasing accessibility to hospital and retail pharmacy channels are further strengthening demand. Early intervention and effective treatment protocols are critical for prolonging gestation, reducing complications, and improving survival rates of preterm infants.
Regulatory Challenges and Safety Concerns Restrain Growth
Market growth is restrained by stringent regulatory requirements for drug approval, safety concerns related to maternal and fetal side effects, and variability in treatment adoption across regions. High costs associated with certain therapies and limited awareness in low-resource settings also restrict penetration.
Additionally, the need for continuous monitoring and specialized healthcare professionals limits large-scale adoption in some markets. These factors are encouraging manufacturers to invest in safer formulations, clinical research, and educational initiatives to drive broader adoption while ensuring compliance and patient safety.
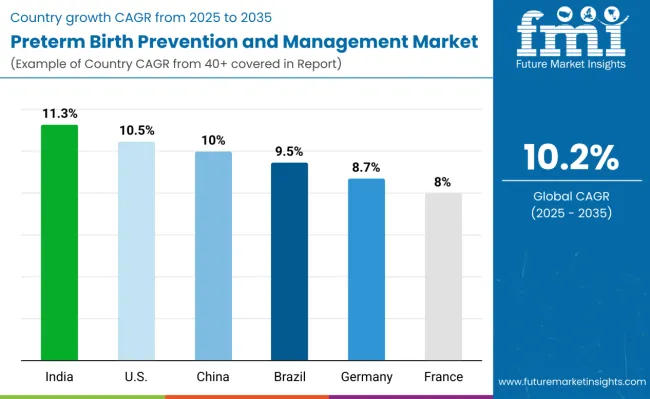
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 11.3% |
| USA | 10.5% |
| China | 10% |
| Brazil | 9.5% |
| Germany | 8.7% |
| France | 8% |
The preterm birth prevention and management market shows varied growth trajectories across the top six countries. India leads with the highest projected CAGR of 11.3% from 2025 to 2035, supported by rising awareness of maternal health, expanding hospital infrastructure, and increasing adoption of progesterone therapy. The USA follows closely at 10.5%, driven by advanced maternal healthcare systems, early screening programs, and high adoption of parenteral therapies.
China maintains steady growth at 10.0%, fueled by rising maternal healthcare investments and growing awareness of neonatal health. Brazil follows at 9.5%, benefiting from government initiatives and expanding hospital and maternal care services. Germany (8.7%) and France (8.0%) experience moderate growth, influenced by established healthcare infrastructure. Overall, these top countries collectively anchor global market expansion through adoption, infrastructure, and healthcare awareness.
The report covers an in-depth analysis of 40+ countries; six top-performing OECD countries are highlighted below.
The preterm birth prevention market in India is projected to expand at a CAGR of 11.3% from 2025 to 2035, driven by rising awareness of maternal health, growing hospital infrastructure, and increasing adoption of progesterone therapy for high-risk pregnancies. Expansion of neonatal care facilities and government programs focused on reducing preterm birth rates are further supporting market growth. Hospitals and maternal care centers are implementing standardized preventive protocols, enhancing early diagnosis and treatment outcomes.
Key Statistics:
Revenue from preterm birth prevention and management in the USA is projected to grow at a CAGR of 10.5% from 2025 to 2035, supported by advanced maternal healthcare infrastructure, widespread screening programs, and strong adoption of progesterone therapy. Parenteral administration remains the preferred route in hospital and clinical settings. Government initiatives, insurance coverage, and maternal health awareness campaigns are contributing to steady demand, while innovations in sustained-release formulations improve patient compliance and outcomes.
Key Statistics:
Sales of preterm birth prevention and management in China are expected to grow at a CAGR of 10.0% from 2025 to 2035, fueled by increased healthcare spending, rising awareness of preterm birth risks, and growing hospital infrastructure. Key urban centers such as Beijing, Shanghai, and Guangzhou are major demand hubs. Clinical adoption of progesterone therapy and parenteral administration is expanding, supported by national maternal health programs and hospital-led initiatives to reduce neonatal mortality.
Key Statistics:
Revenue from preterm birth prevention and management in Brazil is projected to grow at a CAGR of 9.5% from 2025 to 2035, driven by rising hospital deliveries, government initiatives to reduce preterm births, and increased access to maternal healthcare in urban regions. Adoption of progesterone therapy and parenteral delivery is increasing in maternity hospitals. National programs supporting maternal health, combined with urbanization and hospital network expansion, are creating growth opportunities across the country.
Key Statistics:
Demand for preterm birth prevention and management in Germany is expected to grow at a CAGR of 8.7% from 2025 to 2035, supported by a strong healthcare system and well-established obstetric care protocols. Maternal care centers prioritize high-risk pregnancy management with progesterone therapy. Parenteral administration is widely used, while hospitals adopt evidence-based protocols to improve neonatal outcomes. Growth is moderated by already high market penetration and stringent regulatory standards.
Key Statistics:
The preterm birth prevention and management market in France is projected to expand at a CAGR of 8.0% from 2025 to 2035, with adoption focused in urban hospitals and maternal care centers. Progesterone therapy dominates, while parenteral delivery remains the primary administration route. Government-supported maternal health initiatives and clinical protocols aimed at early detection of high-risk pregnancies are supporting steady growth, though market expansion is constrained by established healthcare infrastructure and regulatory oversight.
Key Statistics:
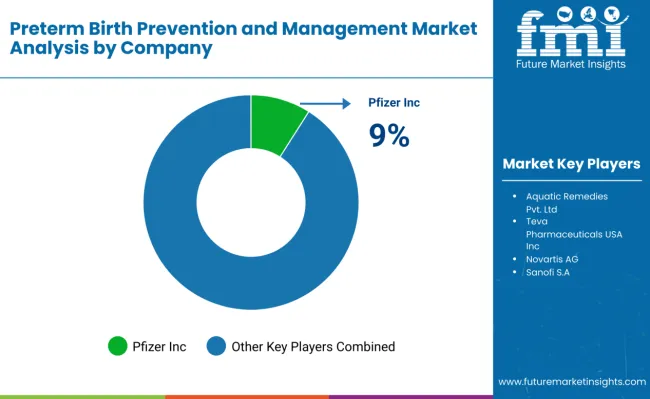
The market is moderately consolidated, comprising multinational pharmaceutical corporations, regional specialty drug manufacturers, and clinical solution providers. Key players include Aquatic Remedies Pvt. Ltd., Teva Pharmaceuticals USA, Inc., Anglo French Drugs & Industries Limited, Biophar Lifesciences Pvt. Ltd., Jasco Labs (P) Ltd., BSA Pharma Inc., Pfizer Inc., Novartis AG, Sanofi S.A. These companies leverage advanced drug formulations, strong R&D capabilities, and extensive distribution networks to supply therapies such as progesterone, corticosteroids, tocolytics, and antihypertensives to hospitals and maternal care centers worldwide.
The market is dominated by progesterone therapy, accounting for over 61.5% of global usage in 2025, particularly in parenteral formulations, which comprise 78.8% of the market. These therapies are preferred for high-risk pregnancies due to their clinical efficacy, patient compliance, and established treatment protocols. Companies are actively focusing on product innovation, including sustained-release formulations, improved safety profiles, and patient-friendly delivery methods, to enhance treatment outcomes and expand market adoption.
Strategic initiatives such as mergers and acquisitions, licensing agreements, partnerships with hospitals, and regional expansions are common among leading players to strengthen market presence. Firms are also investing in clinical research, maternal health programs, and digital solutions to improve therapy accessibility and awareness. Overall, the preterm birth prevention and management market is evolving with established and emerging companies competing through innovation, clinical efficacy, and expanded healthcare outreach, shaping the competitive dynamics of the industry.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 1.9 billion |
| Therapy Type | Progesterone Therapy, Corticosteroid Therapy, Tocolytics Therapy, Antihypertensive Therapy, Magnesium Sulfate Therapy, Heparin Prophylaxis Therapy, Low-Dose Aspirin Therapy, and Antibiotics Therapy |
| Route of Administration | Oral, Parenteral, and Vaginal |
| Patient Type | Prior Spontaneous PTB, Preeclampsia, Short Cervix, Chronic Hypertension, Insulin-dependent, Twins, Antiphospholipid Antibody Syndrome, and Others |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Drug Stores, and Mail Order Pharmacies |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, and Middle East & Africa |
| Country Covered | United States, Canada, United Kingdom, Germany, France, China, Japan, South Korea, Brazil, Australia, and 40+ countries |
| Key Companies Profiled | Aquatic Remedies Pvt. Ltd., Teva Pharmaceuticals USA, Inc., Anglo French Drugs & Industries Limited, Biophar Lifesciences Pvt. Ltd., Jasco Labs (P) Ltd., BSA Pharma Inc., Pfizer Inc., Novartis AG, and Sanofi S.A., |
| Additional Attributes | Dollar sales by therapy type and route of administration; regional adoption trends; competitive landscape; patient preferences for oral versus parenteral therapies; integration with maternal health programs; sustained-release formulation innovations; regulatory compliance adherence |
The global preterm birth prevention and management market is estimated to be valued at USD 1.9 billion in 2025.
The market size for preterm birth prevention and management is projected to reach USD 5 billion by 2035.
The preterm birth prevention and management market is expected to grow at a 10.2% CAGR between 2025 and 2035.
Parenteral administration is projected to lead in the preterm birth prevention and management market with 79% market share in 2025.
Progesterone therapy is projected to dominate in the preterm birth prevention and management market with 61.5% market share in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Preterm Birth Diagnostic Test Kit Market Forecast and Outlook 2025 to 2035
Preterm Births and PROM Testing Market Size and Share Forecast Outlook 2025 to 2035
Birth Tissue Products Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Tax Management Market Size and Share Forecast Outlook 2025 to 2035
Key Management as a Service Market
Cash Management Supplies Packaging Market Size and Share Forecast Outlook 2025 to 2035
Fuel Management Software Market Size and Share Forecast Outlook 2025 to 2035
Risk Management Market Size and Share Forecast Outlook 2025 to 2035
SBOM Management and Software Supply Chain Compliance Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Case Management Software (CMS) Market Size and Share Forecast Outlook 2025 to 2035
Farm Management Software Market Size and Share Forecast Outlook 2025 to 2035
Lead Management Market Size and Share Forecast Outlook 2025 to 2035
Pain Management Devices Market Growth - Trends & Forecast 2025 to 2035
Data Management Platforms Market Analysis and Forecast 2025 to 2035, By Type, End User, and Region
Cash Management Services Market – Trends & Forecast 2025 to 2035
CAPA Management (Corrective Action / Preventive Action) Market
Exam Management Software Market
Asset Management Services Market Size and Share Forecast Outlook 2025 to 2035
Light Management System Market Size and Share Forecast Outlook 2025 to 2035
Labor Management System In Retail Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA