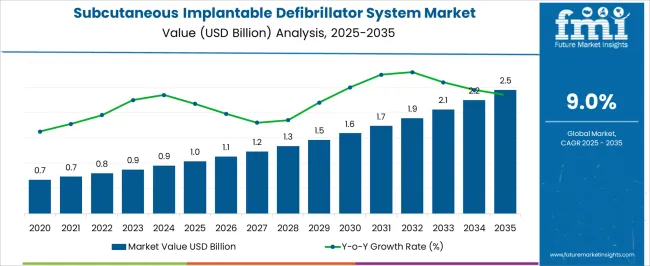
The Subcutaneous Implantable Defibrillator System Market is estimated to be valued at USD 1.0 billion in 2025 and is projected to reach USD 2.5 billion by 2035, registering a compound annual growth rate (CAGR) of 9.0% over the forecast period.
| Metric | Value |
|---|---|
| Subcutaneous Implantable Defibrillator System Market Estimated Value in (2025 E) | USD 1.0 billion |
| Subcutaneous Implantable Defibrillator System Market Forecast Value in (2035 F) | USD 2.5 billion |
| Forecast CAGR (2025 to 2035) | 9.0% |
The subcutaneous implantable defibrillator system market is witnessing steady growth, supported by the rising global prevalence of cardiovascular diseases and the growing adoption of advanced cardiac care technologies. Unlike traditional transvenous systems, subcutaneous devices are being increasingly preferred due to their minimally invasive nature, reduced risk of infection, and avoidance of intravascular leads.
Demand is being further reinforced by advancements in device technology, including improved battery longevity, miniaturization, and enhanced sensing algorithms, which collectively improve patient outcomes and long-term reliability. The market is also being shaped by increasing awareness among both patients and healthcare professionals regarding the benefits of early intervention in sudden cardiac arrest prevention.
Expanding healthcare infrastructure, favorable reimbursement policies, and rising adoption in emerging economies are providing additional growth opportunities As cardiovascular disease burden continues to rise globally, and the need for safer and more effective defibrillation solutions grows, the subcutaneous implantable defibrillator system market is positioned for significant expansion, with manufacturers investing in research and clinical trials to strengthen the efficacy and safety profile of these devices.
The subcutaneous implantable defibrillator system market is segmented by product type, end user, and geographic regions. By product type, subcutaneous implantable defibrillator system market is divided into Single Zone, Dual Zone, and Others. In terms of end user, subcutaneous implantable defibrillator system market is classified into Hospitals, Clinics, Ambulatory Surgical Centers, Cardiac Catheterization Laboratory, and Others. Regionally, the subcutaneous implantable defibrillator system industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
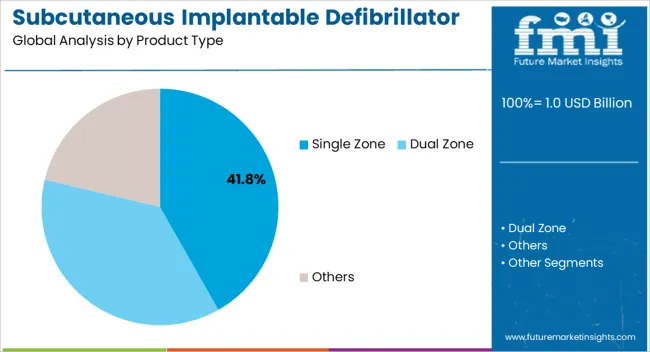
The single zone product type segment is expected to hold 41.8% of the subcutaneous implantable defibrillator system market revenue share in 2025, establishing itself as the leading product category. Its leadership is being driven by strong clinical adoption due to its simplicity, reliability, and effectiveness in treating life-threatening arrhythmias. Single zone systems are designed to detect and deliver therapy efficiently, reducing complexity while maintaining high success rates in sudden cardiac arrest prevention.
Their proven efficacy in both primary and secondary prevention settings has contributed to broad acceptance among physicians and patients. Manufacturing advancements are also supporting their growth, with improved design enabling better sensing accuracy, faster response times, and reduced complication rates compared to earlier models.
The preference for single zone systems is reinforced by their lower cost compared to more complex multi-zone devices, making them more accessible in diverse healthcare settings As the demand for cost-effective, reliable, and safe implantable defibrillator systems continues to rise, the single zone segment is expected to sustain its leadership in the coming years.
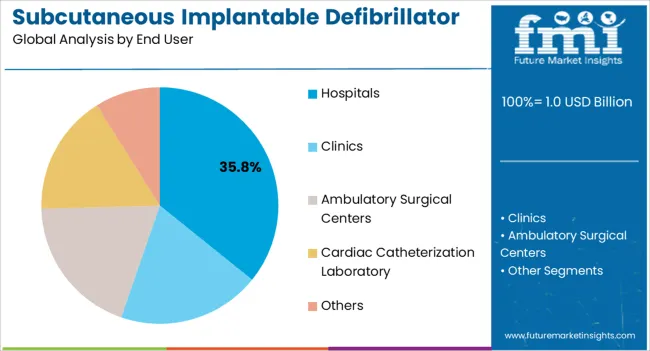
The hospitals end user segment is anticipated to account for 35.8% of the subcutaneous implantable defibrillator system market revenue share in 2025, making it the largest end-use segment. This dominance is being reinforced by hospitals serving as the primary centers for cardiac procedures, where device implantation, monitoring, and follow-up care are most frequently conducted. Hospitals provide access to specialized cardiac electrophysiologists, advanced infrastructure, and emergency care facilities, which are essential for managing complex arrhythmia cases.
The segment is also supported by favorable reimbursement frameworks that encourage patients to seek treatment in hospital settings. As the prevalence of sudden cardiac arrest rises, hospitals are increasingly adopting advanced implantable defibrillator systems to improve survival rates and reduce long-term complications.
Growing investments in hospital-based cardiac care units, particularly in developing regions, are further strengthening the segment’s market share With the continued emphasis on advanced treatment protocols, clinical expertise, and post-procedure monitoring, hospitals are expected to remain the dominant end-use sector in the adoption of subcutaneous implantable defibrillator systems worldwide.
A subcutaneous implantable defibrillator system is a type of defibrillator which continuously monitors the heart rates in cardiac patients. Being placed subcutaneously, it generates electric impulses to treat heart suffering from ventricular tachyarrhythmia and normalized the heart rhythm. Subcutaneous implantable defibrillator system (S-ICD) helps to save patient life by regulating irregular heartbeat. It is generally not used in patient with symptomatic bradycardia. Subcutaneous implantable defibrillator system (S-ICD) consists of titanium case powered with battery and circuit that offers defibrillation therapy, subcutaneous electrodes and accessories.
Subcutaneous implantable defibrillator systems is programmed in order to deliver tier therapy like antitachycardia pacing (ATP) and cardioversion shocks for treating slower hemodynamically stable ventricular tachycardia’s and high energy shocks for treating hemodynamically unstable VT and ventricular fibrillation (VF). Fast ventricular tachycardia are common in cardiac patients. Antitachycardia pacing helps to reduce these episodes by decreasing incidences for syncope. Antitachycardia pacing against fast ventricular tachycardia’s helps to decrease morbidity for shocks.
However, it is generally harmful in patient suffering with symptomatic bradycardia as heart beats too slowly in symptomatic bradycardia patients. The subcutaneous implantable defibrillator system (S-ICD) is implanted without insertion of the leads in the heart.
The subcutaneous implantable defibrillator system is implanted subcutaneous (under the skin) below the armpit along the rib cage or the left axilla and not inserted at the standard location near collar bone. The lead connecting the device is placed under the skin instead of placing it inside the heart.
The advantages of subcutaneous implantable defibrillator systems over other defibrillator are that it provides defibrillation therapy without generating transvenous leads. Moreover, the subcutaneous implantable defibrillator system (S-ICD) do not show any of the symptomatic or long-term complications caused due to implanting a lead in the heart, such as collapse of the lung, perforation of the heart, etc.
Subcutaneous implantable defibrillator systems market is anticipated to be driven by factors like, easy implantable procedure and very less complications.
| Country | CAGR |
|---|---|
| China | 12.2% |
| India | 11.3% |
| Germany | 10.4% |
| France | 9.5% |
| UK | 8.6% |
| USA | 7.7% |
| Brazil | 6.8% |
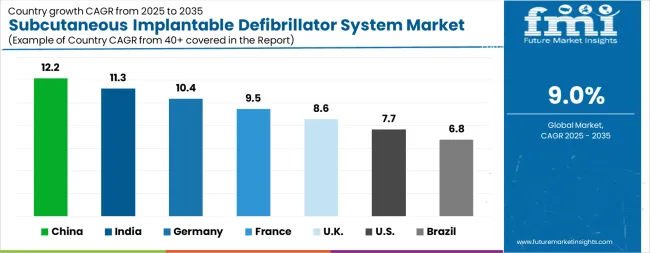
The Subcutaneous Implantable Defibrillator System Market is expected to register a CAGR of 9.0% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 12.2%, followed by India at 11.3%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 6.8%, yet still underscores a broadly positive trajectory for the global Subcutaneous Implantable Defibrillator System Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 10.4%. The USA Subcutaneous Implantable Defibrillator System Market is estimated to be valued at USD 360.4 million in 2025 and is anticipated to reach a valuation of USD 753.2 million by 2035. Sales are projected to rise at a CAGR of 7.7% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 52.1 million and USD 35.1 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 1.0 Billion |
| Product Type | Single Zone, Dual Zone, and Others |
| End User | Hospitals, Clinics, Ambulatory Surgical Centers, Cardiac Catheterization Laboratory, and Others |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
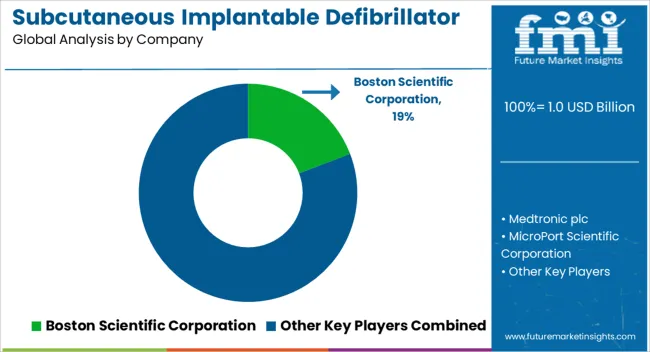
| Key Companies Profiled | Boston Scientific Corporation, Medtronic plc, MicroPort Scientific Corporation, LivaNova PLC Company, Imricor Medical Systems, Inc., MRI Interventions, Inc, and Mayo Clinic US |
The global subcutaneous implantable defibrillator system market is estimated to be valued at USD 1.0 billion in 2025.
The market size for the subcutaneous implantable defibrillator system market is projected to reach USD 2.5 billion by 2035.
The subcutaneous implantable defibrillator system market is expected to grow at a 9.0% CAGR between 2025 and 2035.
The key product types in subcutaneous implantable defibrillator system market are single zone, dual zone and others.
In terms of end user, hospitals segment to command 35.8% share in the subcutaneous implantable defibrillator system market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Subcutaneous Drug Delivery Market Size and Share Forecast Outlook 2025 to 2035
Market Share Insights for Subcutaneous Drug Delivery Devices Providers
Subcutaneous Biologics Market
Subcutaneous Implantable Defibrillator (S-ICD) Market is segmented by product, indication, and end user from 2025 to 2035
Implantable Tibial Neuromodulation Market Forecast and Outlook 2025 to 2035
Implantable Collamer Lens Market Size and Share Forecast Outlook 2025 to 2035
Implantable Infusion Pump Market Size and Share Forecast Outlook 2025 to 2035
Implantable Drug Eluting Devices Market Size and Share Forecast Outlook 2025 to 2035
Implantable Drug Infusion Pumps Market
Implantable Defibrillator Market Size and Share Forecast Outlook 2025 to 2035
System-On-Package Market Size and Share Forecast Outlook 2025 to 2035
Systems Administration Management Tools Market Size and Share Forecast Outlook 2025 to 2035
Systemic Sclerosis Treatment Market - Trends & Forecast 2025 to 2035
System on Module Market Growth – Trends & Forecast 2025 to 2035
SLE Drugs Market Insights - Growth & Forecast 2025 to 2035
Systemic Mastocytosis Treatment Market
Systemic Infection Treatment Market
5G System Integration Market Insights - Demand & Growth Forecast 2025 to 2035
VRF Systems Market Growth - Trends & Forecast 2025 to 2035
Rail System Dryer Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA