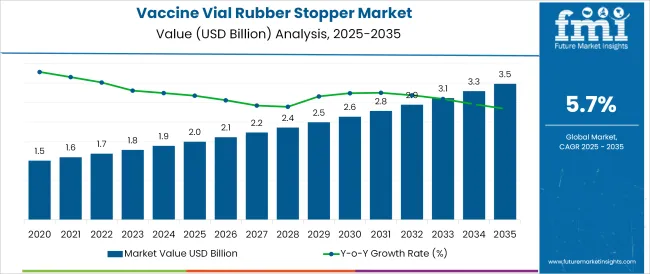
The Vaccine Vial Rubber Stopper Market is estimated to be valued at USD 2.0 billion in 2025 and is projected to reach USD 3.5 billion by 2035, registering a compound annual growth rate (CAGR) of 5.7% over the forecast period.
The vaccine vial rubber stopper market is witnessing measured yet sustained growth, underpinned by global immunization initiatives, rising demand for injectable biologics, and stringent safety regulations in pharmaceutical packaging. Growth is being supported by advancements in elastomeric formulations that ensure chemical compatibility, minimize extractables, and maintain sterility throughout storage and transport.
The push for precision drug delivery and vaccine efficacy has necessitated enhanced barrier properties and tight container-closure integrity, driving innovation in stopper designs. Regulatory bodies across the U.S., EU, and APAC have emphasized compliance with cGMP standards, resulting in increased adoption of high-quality closures.
Furthermore, the rise in vaccine production, including mRNA-based platforms and multi-dose vials, is fueling demand for stoppers that can withstand frequent punctures and preserve sterility. The market outlook remains favorable, particularly as governments and healthcare institutions invest in cold chain infrastructure and clinical rollout of next-generation vaccines, creating long-term opportunities for material innovation and manufacturing scalability.
The market is segmented by Based on Size, Based on Material, and Based on End Use and region. By Based on Size, the market is divided into 13 mm, 20 mm, 28 mm, and 32 mm. In terms of Based on Material, the market is classified into Butyl Rubber, Chlorobutyl Rubber, and Bromobutyl Rubber.
Based on Based on End Use, the market is segmented into Medical & Healthcare, Pharmaceutical, and Research & Development. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
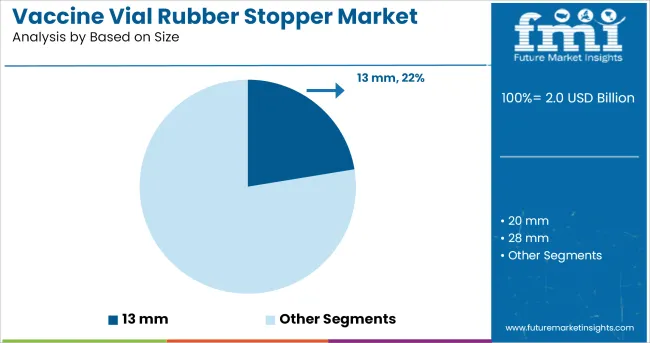
It is projected that the 13 mm size segment will account for 22.40% of total market revenue by 2025, making it one of the leading stopper sizes in use. This preference is primarily driven by its widespread application in small-volume vaccine vials, particularly those used for single-dose administration.
The compact size has been favoured for ease of integration with standardized glass vial formats and compatibility with automated filling lines. Its smaller surface area contributes to a reduced risk of contamination and extractables, which is critical for sensitive formulations.
In cold storage and transportation, the 13 mm stoppers have demonstrated effective sealing performance, preserving vial integrity under varying thermal conditions. These functional attributes, combined with global demand for individual-dose vaccines, have solidified the 13 mm format as a staple in the vaccine packaging segment.
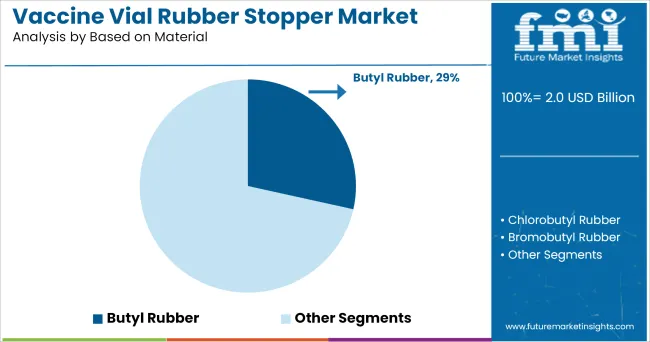
The butyl rubber segment is anticipated to hold 28.50% of total market revenue, emerging as the most preferred material type in 2025. Its dominance is attributed to exceptional chemical resistance, low permeability to gases, and strong resealability after multiple needle punctures.
These characteristics have made butyl rubber an industry standard for vaccine applications, particularly where sterility and long-term stability are non-negotiable. Additionally, its formulation compatibility with a wide range of biologics and conventional vaccines has ensured regulatory acceptance and global scalability.
Enhanced processing techniques have further improved its physical strength and reduced the presence of leachables. As a result, butyl rubber continues to be the material of choice for vaccine vial stoppers, driven by its proven reliability, safety profile, and compliance with international pharmacopeial standards.
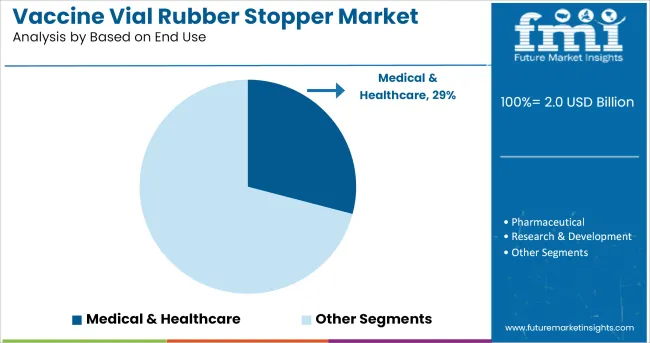
It is expected that the medical and healthcare segment will account for 29.10% of total market revenue by 2025, making it the primary end-use category. This is driven by large-scale immunization programs, expanding clinical trials, and increasing reliance on parenteral therapies across hospitals and vaccination centers.
The healthcare sector’s adherence to sterility standards, cold chain protocols, and packaging validation procedures has reinforced the use of precision-engineered stoppers.
Moreover, institutional procurement from government agencies and global health organizations has favored suppliers capable of delivering consistent, regulatory-compliant components at scale. This sustained demand across clinical and commercial supply chains has positioned the medical and healthcare sector as the most significant consumer of vaccine vial rubber stoppers.
Packaging plays a vital role in vaccine protection, transportation, and distribution. Vial sealing is a critical process that ensures sterility and leakage prevention of the final product.
Vaccine vial rubber stoppers are good in airtightness, heat resistance, alkaline and acid resistance, and high internal cleanliness. The vaccine vial rubber stoppers maintain better chemical stability and quality of the medicine. These vaccine vial stoppers are made of high-quality rubber material, which is washable, durable, reusable, and resistant to high and low temperatures. They create a significant barrier that extends the life of the material inside the bottle.
The vaccine vial rubber stoppers protect the medicines from environmental impacts, bottle closure integrity, and safety. The rubber stoppers for vaccine vials must have specific properties to seal the vaccine at high ambient and extremely low temperatures. Thus, rubber stoppers seal the vaccine doses in vials and have been mobilized as part of the global fight against the coronavirus.
With the skyrocketing demand for the vaccination around the globe, the need for vaccine vial rubber stoppers has increased tremendously. Additionally, the increasing demand for lyophilisation also raises the demand for the vaccine vial rubber stopper market.
The vaccine vial rubber stoppers have stable chemical properties, good biological safety, no pyrogenous substance, and physiological toxicity. Additionally, they have good drug compatibility and don’t absorb and mitigate when directly contacting the liquid medicines.
They avoid entering any impurities and contaminant-free protection and increase the drugs’ shelf life, leading to market growth. The vaccine vial rubber stopper market is witnessing high growth because it provides a perfect barrier against moisture, gases, and UV rays. Thus, the vaccine vial rubber stopper is anticipated a high growth.
Producing and supplying the millions of vaccine doses required enormous quantities of vaccine vial rubber stoppers, which few companies manufactured.
Limited production capacity, shortage of personnel, scarcity of raw material, lack of coordination, and massive manufacturing effort to produce the vaccine vial rubber stopper at an accelerated speed to cut delivery time were some of the challenges that could affect the growth of the product. The rubber stopper for vaccine doses requires Government approval as per specifications.
Therefore, the task of manufacturing billions of rubber stoppers within a few months lead time and as per the specific properties with sterilization to make it available to the public as quickly as possible could restrain the growth of the vaccine vial rubber stopper market.
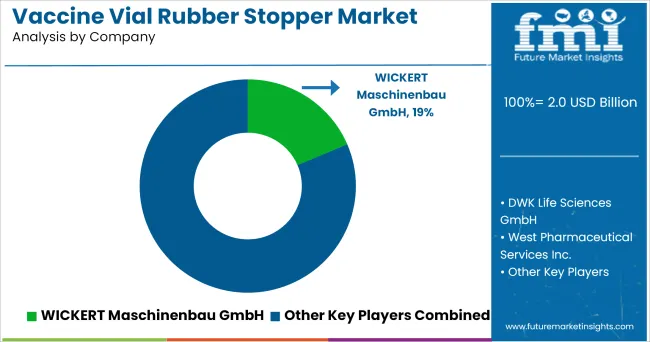
Key players such as
are actively involved in vaccine vial rubber stopper market for different applications.
Key Asian players such as
are actively involved in vaccine vial rubber stopper market for different applications.
The manufacturers involved in manufacturing vaccine vial rubber stoppers are coming up with innovations and adopting various strategies such as product launches to serve the increasing demand for the vaccine rubber stopper market.
India is a developing country with a vast population; several vaccinations made mandatory by the Indian Government to protect infants and adults from harmful diseases. The top vaccinations such as BCG vaccine, DPT vaccine, Oral Polio vaccine, MMR vaccine, Hepatitis B vaccine are administered to the babies at birth.
Additionally, the highest growth of the pharmaceutical sector, increasing research and development in the medical field, increasing the number of chemical and pathological laboratories, raising awareness of the people towards health and hygiene.
The global vaccine vial rubber stopper market is estimated to be valued at USD 2.0 billion in 2025.
The market size for the vaccine vial rubber stopper market is projected to reach USD 3.5 billion by 2035.
The vaccine vial rubber stopper market is expected to grow at a 5.7% CAGR between 2025 and 2035.
The key product types in vaccine vial rubber stopper market are 13 mm, 20 mm, 28 mm and 32 mm.
In terms of based on material, butyl rubber segment to command 28.5% share in the vaccine vial rubber stopper market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Vaccine Preservatives Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Vaccine Stabilizers Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Vaccine Transport Carrier Market Size and Share Forecast Outlook 2025 to 2035
Vaccine Shippers Market Size and Share Forecast Outlook 2025 to 2035
Vaccines Market Insights - Trends, Growth & Forecast 2025 to 2035
Vaccine Packaging Market Growth - Demand & Forecast 2024 to 2034
Vaccine Ampoules Market
Dog Vaccine Market Size and Share Forecast Outlook 2025 to 2035
Cat Vaccines Market Size and Share Forecast Outlook 2025 to 2035
Fish Vaccines Market
Live Vaccines Market
Swine Vaccine Market Size and Share Forecast Outlook 2025 to 2035
Covid Vaccine Packaging Market Size and Share Forecast Outlook 2025 to 2035
Market Share Distribution Among Covid Vaccine Packaging Manufacturers
Nasal vaccines Market
Travel Vaccines Market Size and Share Forecast Outlook 2025 to 2035
Cancer Vaccines Market Analysis by Technology, Treatment Method, Application and Region from 2025 to 2035
Dengue Vaccines Analysis by Product Type by Product, By Age Group and by Distribution Channel through 2035
COVID-19 Vaccine Packaging & Delivery Devices Market - Innovations & Trends 2025 to 2035
Covid-19 Vaccine Development Tools Market - Growth & Forecast 2024 to 2034

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA