The Global Digital Twins Technologies Market has been forecasted to attain a valuation of USD 601.8 million in 2025, rising to USD 1,771.35 million by 2035, resulting in an incremental gain of USD 1,169.5 million, which reflects a 34.0% increase over the forecast decade. A compound annual growth rate (CAGR) of 11.4% is expected to be recorded, indicating a rapid expansion trajectory for the global digital twins technologies market.
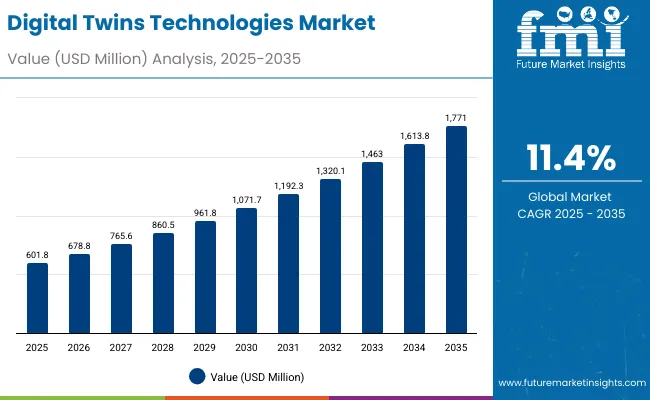
Global Digital Twins Technologies Market Key Takeaways
| Metric | Value |
|---|---|
| Global Digital Twins Technologies Market Estimated Value in (2025E) | USD 601.8 million |
| Global Digital Twins Technologies Market Forecast Value in (2035F) | USD 1,771 . 3 million |
| Forecast CAGR (2025 to 2035) | 11.4% |
Between 2025 and 2030, digital twins technologies will see broader adoption across healthcare, as hospitals, research institutions, and medical device companies increasingly rely on real-time patient simulations and predictive analytics to optimize treatment plans, anticipate health events, reduce clinical risks, and improve medical device design and operational efficiency.
It will grow upto USD 430.7 million in that period from USD 601.8 million in 2025 to USD 1,032.5 million in 2030, driven by the integration of IoT sensors, edge computing, and cloud-based analytics. The convergence of high-fidelity simulation platforms with user-friendly visualization tools is making digital twins more accessible, prompting uptake even among mid-tier industrial enterprises and regional smart city projects.
Between 2030 and 2035, the market is projected to expand further by USD 738.9million, fueled by advances in AI-driven predictive modeling, augmented reality interfaces, and real-time data synchronization. Demand will rise from sectors such as autonomous manufacturing, renewable energy management, and urban infrastructure planning, where predictive maintenance and scenario testing are critical.
Continuous development in high-speed connectivity, digital twin interoperability standards, and multi-physics modeling will shape the next generation of digital twin platforms.
From 2020 to 2024, the global digital twins technologies market, expanded from USD 321.4million to USD 542.8 million, driven by broader availability of cloud-based solutions, simulation software, and real-time monitoring tools. During this period, technology providers partnered with enterprises and research institutions to simplify integration, optimize platform performance, and standardize analytics workflows laying the foundation for wider adoption across manufacturing, energy, and urban development sectors.
Looking forward, the digital twins ecosystem is likely to evolve to incorporate AI-powered anomaly detection, multi-domain system modeling, and hybrid cloud-edge deployment. These innovations aim to reduce the complexity of deployment, expand interdisciplinary usability, and improve operational efficiency making digital twins an essential tool in industrial automation, smart infrastructure, and product lifecycle management.
The growth of the global digital twins technologies market is reinforced by the unique ability of digital twin platforms to create highly accurate virtual replicas of physical assets, processes, and systems enabling real-time monitoring, predictive analytics, and performance optimization.
Unlike traditional healthcare modeling tools, digital twins provide dynamic, data-driven insights into patient physiology, treatment responses, and system interdependencies, making them indispensable for next-generation personalized medicine, hospital workflow optimization, and medical device lifecycle management.
Over the last decade, the digital twinstechnologies becoming critical tools in healthcare, enabling detailed analysis of patient physiology, hospital operations, and medical device performance. They support predictive diagnostics, personalized treatment planning, and simulation of clinical interventions at scale.
As hospitals and research institutions increasingly adopt IoT-enabled medical devices, cloud computing, and AI-driven analytics, digital twins are emerging as an essential component of healthcare digital transformation strategies, improving patient outcomes, operational efficiency, and the development of next-generation therapies and medical technologies.
Another factor driving adoption is the shift from complex, on-premises deployments to highly capable cloud-based and hybrid digital twin platforms. With technology providers now offering turnkey solutions that integrate real-time sensor data, multi-physics simulation, and AI-powered predictive models, organizations can achieve high-fidelity insights without extensive in-house infrastructure. This decentralization is transforming the accessibility of digital twins, particularly for mid-sized enterprises and emerging market applications.
Moreover, increasing demand from interdisciplinary healthcare domains including hospital operations, medical device development, and personalized medicine is fostering the development of hybrid digital twin modalities that combine physiological simulations with AI-driven predictive analytics. These systems address the growing need to optimize treatment plans, anticipate patient health events, reduce clinical risks, and support real-time decision-making in complex, dynamic healthcare environments.
Collectively, these technological and operational drivers are positioning the healthcare digital twins market for sustained expansion, fueled by its essential role in enabling predictive, efficient, and resilient patient care, hospital workflows, and medical device lifecycle management.
The market is segmented by type, application, and end user. By type, the market includes Patient Digital Twins, Device Digital Twins, Facility Digital Twins, Process Digital Twins, and Others. By application, the segmentation covers Personalized Medicine, Drug Discovery & Pre-clinical/Virtual Trials, Surgical Planning & Simulation, Medical Device Design & Testing, Workflow & Operational Optimization, Patient Monitoring, and Others.
Based on end user, the market comprises Hospitals, Pharma & Biopharma Companies, Medical Device Manufacturers, Research & Academic Institutions, Clinical Research Organizations (CROs), and others.Regionally, the scope spans North America, Latin America, Western and Eastern Europe, East Asia, South Asia and Pacific, and the Middle East and Africa.
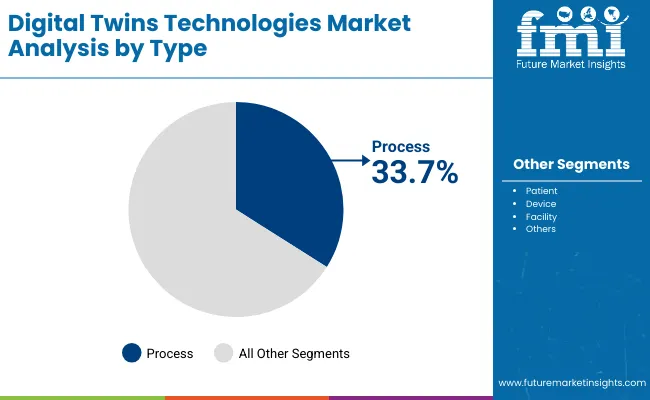
| Type of Digital Twin | Market Value Share, 2025 |
|---|---|
| Patient | 18.2% |
| Device | 25.3% |
| Facility | 15.0% |
| Process | 33.7% |
| Others | 7.8% |
Process Digital Twins are projected to hold the largest share in the healthcare digital twins market at 33.7% in 2025. These models replicate hospital workflows, clinical operations, and supply chain processes, enabling administrators and clinicians to optimize efficiency, reduce bottlenecks, and improve patient throughput.
By integrating real-time data from electronic health records, IoT-enabled medical devices, and hospital management systems, process digital twins allow simulation of operational scenarios, predictive analysis, and resource planning.
This helps healthcare facilities anticipate demand surges, minimize downtime, and enhance overall care quality. Compared to Patient, Device, or Facility digital twins, process models focus on systemic optimization across departments, ensuring cost-effective, safe, and streamlined operations. Their dominant market share reflects the growing need for scalable, data-driven solutions to manage complex healthcare workflows.
Recent advances in IoT-enabled sensors, cloud-based analytics, and multi-scale simulation platforms have enabled these digital twins to operate at near-real-time performance, providing actionable insights throughout the device lifecycle. Moreover, integration with workflow management systems and patient monitoring data allows organizations to assess device performance in clinical and operational contexts making them indispensable for medical device R&D and hospital deployment.
A key factor behind their dominance is the growing adoption across medical device manufacturers, hospitals, and research institutions. With solution providers offering turnkey platforms that require minimal technical overhead, device digital twins are becoming increasingly accessible to mid-sized enterprises and emerging market players. Their relatively lower deployment complexity and high ROI further position them as the go-to solution for optimizing device performance, safety, and lifecycle management.
As healthcare systems continue to emphasize efficiency, safety, and predictive capabilities, the precision, scalability, and interoperability of device digital twins ensure that they remain the backbone of modern digital twin applications across hospitals, manufacturers, and research labs alike.
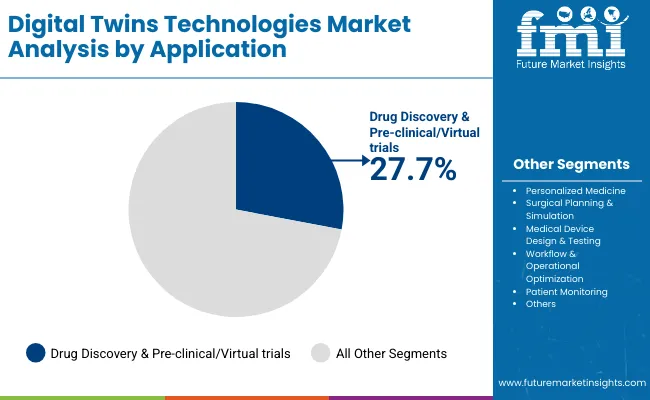
| Application | Market Value Share, 2025 |
|---|---|
| Personalized Medicine | 24.3% |
| Drug Discovery & Pre-clinical/Virtual trials | 27.7% |
| Surgical Planning & Simulation | 9.0% |
| Medical Device Design & Testing | 11.6% |
| Workflow & Operational Optimization | 13.0% |
| Patient Monitoring | 8.2% |
| Others | 6.2% |
Drug Discovery & Pre-clinical/Virtual Trials continues to dominate the application landscape, accounting for 27.7% of total demand in 2025. This application remains the foundation of digital twin adoption in healthcare due to its proven ability to simulate biological systems, predict drug interactions, and optimize trial designs with high accuracy and efficiency. As an essential tool in pharmaceutical R&D, digital twins offer unmatched insight into molecular behavior, therapeutic efficacy, and patient response variability.
What makes digital twins indispensable in drug discovery is their reproducibility, standardization, and integration with existing clinical and molecular datasets. Many organizations have developed extensive expertise with pre-clinical and virtual trial simulations, building large repositories of modeled outcomes that create a rich framework for comparative studies. The approach is particularly effective in modeling complex diseases, optimizing compound selection, and reducing time-to-market for new therapeutics.
Recent improvements in AI-driven modeling, high-performance computing, and real-time data integration have further enhanced the accuracy and usability of digital twins in pharmaceutical development. Moreover, academic programs and corporate research initiatives continue to prioritize digital twin simulations as primary training and experimental tools, ensuring a sustained pipeline of skilled users and continued investment.
While other applications such as Personalized Medicine, Workflow & Operational Optimization, and Surgical Planning are gaining traction in niche use cases, drug discovery and pre-clinical/virtual trials remain the mainstay of the market, largely due to their critical role in reducing R&D costs, accelerating innovation, and improving the predictability of clinical outcomes.
The adoption of digital twins is accelerating across multiple healthcare and industrial applications as sensor technology, AI, and simulation platforms converge to deepen operational insights and predictive capabilities. While momentum is strong, the market also faces structural limitations unique to deployment complexity and data integration requirements.
Growing Role in Healthcare, Manufacturing, and InfrastructureDigital twins are becoming essential in healthcare, particularly in personalized medicine, medical device lifecycle management, and hospital workflow optimization. Unlike conventional monitoring and simulation tools, they provide dynamic, data-driven insights into patient physiology, operational behavior, system interactions, and predictive failure points, enabling clinicians and administrators to make more informed decisions.
Hospitals and research institutions are using digital twins to simulate treatment outcomes, optimize care pathways, and validate medical device designs, improving both patient safety and operational efficiency. Integration with IoT-enabled devices and AI-driven analytics allows real-time monitoring, predictive diagnostics, and proactive intervention, which conventional tools cannot achieve.
As adoption grows, digital twins are emerging as standard tools in clinical decision-making, process optimization, and medical device testing, supporting evidence-based, data-driven workflows. This convergence of AI, IoT, and simulation is positioning digital twins as a critical component of modern healthcare infrastructure and digital transformation strategies.
Shift Toward Cloud-Based and Turnkey PlatformsA key trend in the healthcare digital twins market is the growing adoption of cloud-native and hybrid platforms. These platforms integrate real-time patient data, predictive analytics, and visualization dashboards, enabling clinicians and administrators to simulate physiological responses, optimize treatment plans, and monitor medical device performance.
Advances in AI, IoT-enabled medical devices, and edge computing allow healthcare providers to implement digital twins without investing in large IT infrastructures. User-friendly interfaces are making these solutions accessible to a wider range of healthcare professionals, including those in smaller hospitals and mid-sized research centers. Turnkey solutions are increasingly deployed to improve patient outcomes, streamline hospital workflows, and enhance operational efficiency.
This shift toward scalable, data-driven, and interoperable platforms is accelerating the adoption of digital twins beyond early technology adopters. Overall, the trend reflects a move toward predictive, personalized, and efficient healthcare delivery, positioning digital twins as a standard tool in healthcare digital transformation initiatives.
Infrastructure, Expertise, and Data ConstraintsDigital twins in healthcare require extensive data collection, integration of heterogeneous systems, and advanced analytics capabilities, making their setup and maintenance resource-intensive. Ensuring high-quality data, real-time synchronization, and accurate model validation is critical, as errors can compromise predictive accuracy and patient safety.
Effective deployment demands expertise in IoT-enabled medical devices, AI-driven analytics, simulation software, and domain-specific clinical workflows, creating a significant training and adoption bottleneck. These challenges particularly affect smaller hospitals, clinics, and research institutions with limited digital infrastructure, restricting the scalability of digital twin solutions. Even advanced platforms can remain underutilized without adequate personnel, IT resources, and institutional support.
Consequently, the complexity, technical skill requirements, and operational demands represent a key restraint on the healthcare digital twins market, limiting broader adoption. Addressing these barriers through turnkey solutions, standardized protocols, and targeted training programs will be essential for expanding the market beyond well-funded healthcare organizations and large enterprises.
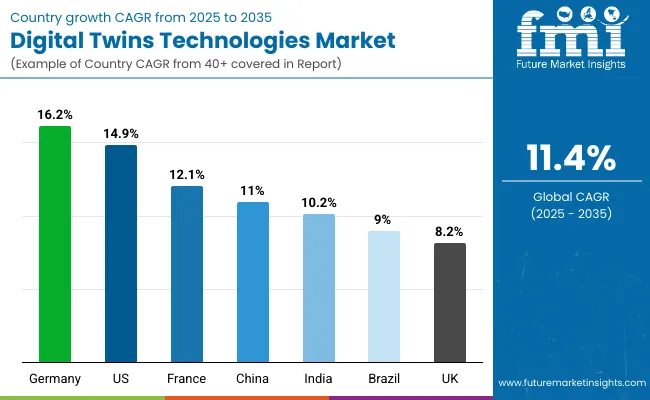
| Country | CAGR |
|---|---|
| USA | 14.9% |
| Brazil | 9.0% |
| China | 11.0% |
| India | 10.2% |
| Europe | 12.1% |
| Germany | 16.2% |
| France | 12.1% |
| UK | 8.2% |
The adoption and growth patterns of digital twin technologies exhibit distinct variations across different regions, influenced by the maturity of IT and healthcare infrastructure, government and private investment in advanced analytics, and specialized application demand.
In the United States, the digital twins market is characterized by strong growth driven by a robust ecosystem of hospitals, research institutions, and technology companies focused on healthcare innovation, smart manufacturing, and operational optimization. The availability of high-speed connectivity, cloud computing platforms, and AI-powered simulation tools supports advanced digital twin applications, resulting in wide spread adoption.
The presence of specialized innovation centers and continuous investments in predictive analytics, device lifecycle management, and virtual modeling propels demand for digital twin solutions, particularly in areas such as personalized medicine, drug discovery, and workflow optimization. The market here is projected to account for 14.9% of the global digital twins technologies market.
In Europe, countries such as Germany, France, and the UK lead the digital twins landscape with growing emphasis on integrating digital twin technologies within national healthcare, manufacturing, and smart infrastructure initiatives.
Government-backed programs and industry-academia collaborations have simplified adoption, enabling the use of AI-driven, cloud-based, and hybrid digital twin platforms for advanced applications such as personalized medicine, device lifecycle management, and operational optimization.
Regulatory support for digital infrastructure and strategic funding foster sustained market growth, though adoption remains cautiously progressive as new solutions continue to prove their value in real-world environments. The market in Germany is projected to account for 13.4% of the global digital twins market, France 10.0%, and the UK 8.2%.
Overall, the digital twins market globally reflects a strategic blend of technological advancement, policy alignment, and institutional investment, which together govern the pace and scale of adoption in different countries. This dynamic interplay underscores the central role that regional infrastructure, funding support, and technical expertise play in shaping the trajectory of this transformative technology.
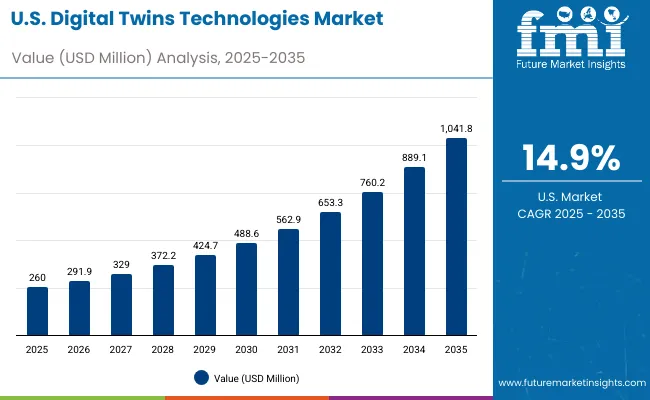
The digital twins technologies market in the United States has been forecasted to expand at a CAGR of 14.9% between 2025 and 2035. The USA remains the most mature and technology-intensive market for digital twins, with widespread integration across healthcare, pharma, and industrial applications.
Companies like Philips Healthcare, Microsoft, and Twin Health have played a central role in advancing digital twin adoption in personalized medicine, medical device lifecycle management, and healthcare operations optimization.
The United States also hosts some of the world’s most advanced cloud platforms, IoT infrastructures, and AI analytics systems, supporting real-time patient monitoring, predictive modeling, and high-fidelity simulations that enhance clinical decision-making and operational efficiency.
The digital twins technologies market in India is expected to grow at a CAGR of 10.2% between 2025 and 2035, with growing awareness but limited infrastructure outside top-tier institutions. Leading organizations, including AIIMS, IIT Bombay, and IIT Delhi, along with top pharmaceutical R&D centers, are exploring digital twin applications in personalized medicine, device evaluation, and hospital operations. Despite this progress, fully operational deployments are limited due to high initial investment requirements, scarce cloud and IoT expertise, and dependence on government funding.
China’s digital twins technologies market is projected to expand at a CAGR of 11.0% from 2025 to 2035. China’s market is expanding rapidly, driven by strong government initiatives in smart healthcare, industrial IoT, and urban infrastructure. Top-tier universities such as Tsinghua and Peking University, together with the Chinese Academy of Sciences, have developed advanced digital twin systems for healthcare, device lifecycle, and operational optimization.
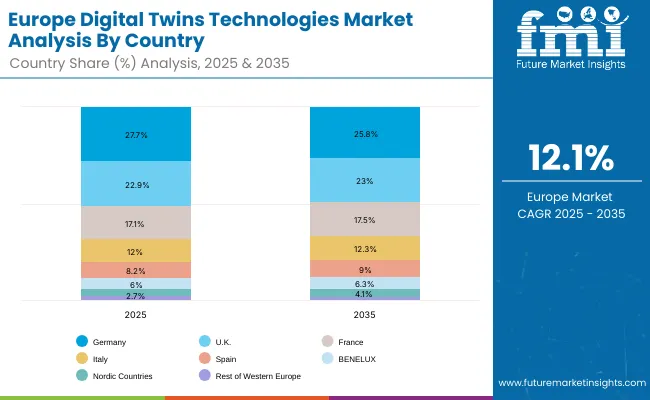
| Europe Country | 2025 |
|---|---|
| Germany | 27.7% |
| UK | 22.9% |
| France | 17.1% |
| Italy | 12.0% |
| Spain | 8.2% |
| BENELUX | 6.0% |
| Nordic Countries | 3.4% |
| Rest of Western Europe | 2.7% |
| Europe Country | 2035 |
|---|---|
| Germany | 25.8% |
| UK | 23.0% |
| France | 17.5% |
| Italy | 12.3% |
| Spain | 9.0% |
| BENELUX | 6.3% |
| Nordic Countries | 4.1% |
| Rest of Western Europe | 2.0% |
The digital twins technologies market in the United Kingdom is projected to grow at a CAGR of 8.2% between 2025 and 2035. The UK market is supported by strong public and private investment in healthcare innovation, pharma R&D, and industrial operational optimization.
NHS Trusts are creating digital twins to simulate pandemic response scenarios, test hospital staffing policies, and model vaccination rollout strategies, allowing policymakers to anticipate challenges and optimize resource allocation before real-world implementation.
The UK is positioning digital twins primarily as a strategic tool for governance, compliance, and public health planning, rather than purely operational or clinical applications. The country’s emphasis is on standardization, regulation, and evidence-based decision-making.
The digital twins technologies market in Germany is anticipated to grow at a CAGR of 13.4% between 2025 and 2035. Germany is positioning itself as a key hub for high-precision digital twin applications, particularly in healthcare, pharma, and industrial automation.
While academic research remains strong, much of the current growth is fueled by collaborations between hospitals, manufacturing companies, and technology vendors. German firms are playing a strategic role in developing digital twin subsystems including AI analytics platforms, IoT-enabled sensor networks, and cloud-based simulation environments which are now being integrated into national and international workflows.
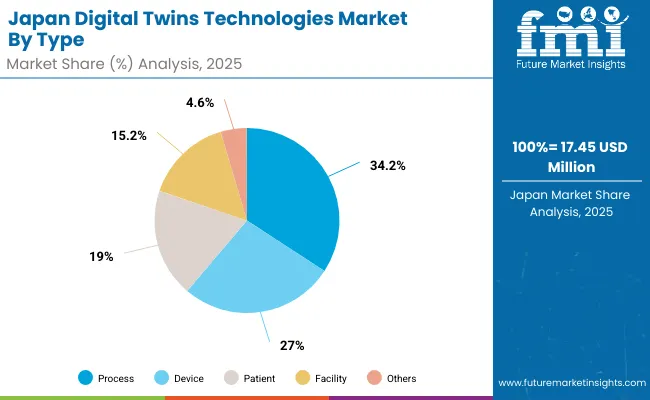
| By Type of Digital Twin | Market Value Share, 2025 |
|---|---|
| Patient | 19.0% |
| Device | 27.0% |
| Facility | 15.2% |
| Process | 34.2% |
| Others | 4.6% |
The digital twins technologies market in Japan has been projected to reach USD 17.45 million by 2025. Device-based digital twin platforms are expected to lead the product landscape with a 27.0% share, followed by process-focused twins at 34.2% and patient-centric solutions at 19.0%.
The market in Japan is shaped by a long-standing tradition of advanced healthcare technology adoption and industrial innovation. Japan has oriented its digital twin initiatives toward high-precision clinical, biomedical, and translational research applications, emphasizing personalized medicine, surgical accuracy, and drug development.
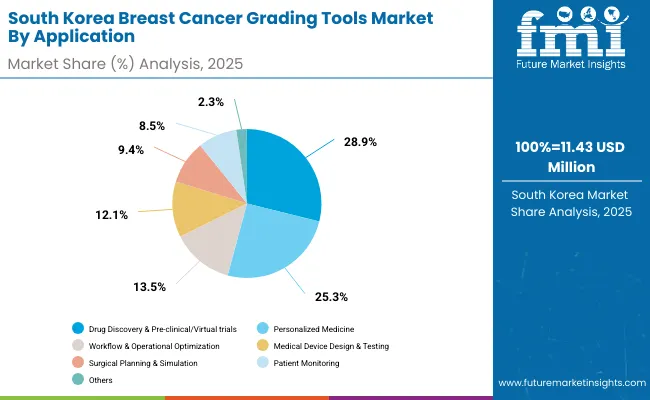
| By Application | Market Value Share, 2025 |
|---|---|
| Personalized Medicine | 25.3% |
| Drug Discovery & Pre-clinical/Virtual trials | 28.9% |
| Surgical Planning & Simulation | 9.4% |
| Medical Device Design & Testing | 12.1% |
| Workflow & Operational Optimization | 13.5% |
| Patient Monitoring | 8.5% |
| Others | 2.3% |
The digital twins technologies market in South Korea has been projected to reach USD 11.43 million in 2025. Drug discovery and pre-clinical/virtual trials are expected to lead the application landscape with a 28.9% share, followed by personalized medicine at 25.3%. South Korea leverages digital twins not just in healthcare but as a tool for industrial efficiency, logistics optimization, and cross-sector integration, connecting hospitals, manufacturing, and smart city infrastructure.
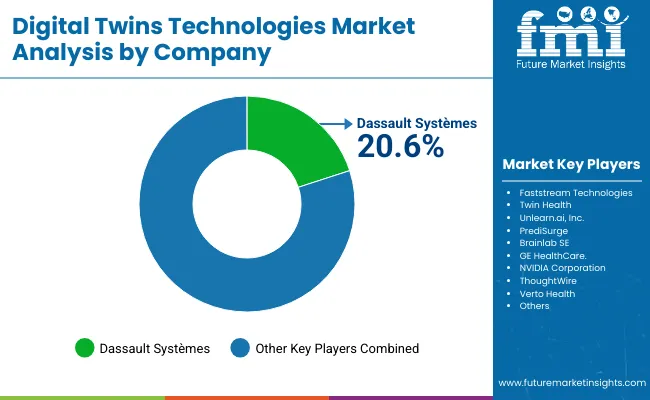
| Company | Global Value Share 2024 |
|---|---|
| Dassault Systèmes | 20.6% |
| Others | 79.4% |
The global autonomous radiology systems market is moderately concentrated, with DassaultSystèmes holding a leading position due to its comprehensive AI-driven healthcare platform and integration across imaging, surgical planning, and diagnostic workflows. The company’s dominance is supported by its ability to provide end-to-end solutions combining software, cloud infrastructure, and interoperability with hospital IT systems. DassaultSystèmes accounted for 20.6% of the global market in 2024.
Other notable players collectively accounting for 79.4% of the market include Faststream Technologies, Twin Health, Unlearn.ai, Inc., PrediSurge, Brainlab SE, GE HealthCare, NVIDIA Corporation, ThoughtWire, and Verto Health.
Key Developments in Global Digital Twins Technologies Market
| Item | Value |
|---|---|
| Quantitative Units | USD 601.8 million |
| By Type of Digital Twin | Patient, Device, Facility, Process, Others |
| By Application | Personalized Medicine, Drug Discovery & Pre-clinical/Virtual Trials, Surgical Planning & Simulation, Medical Device Design & Testing, Workflow & Operational Optimization, Patient Monitoring, Others |
| By End User | Hospitals, Pharma & Biopharma Companies, Medical Device Manufacturers, Research & Academic Institutions, Clinical Research Organizations (CROs), Others |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa |
| Countries Covered | USA, Brazil, China, India, Europe, Germany, France and UK |
| Key Companies Profiled | Dassault Systèmes , Faststream Technologies, Twin Health, Unlearn.ai, Inc., PrediSurge , Brainlab SE, GE HealthCare, NVIDIA Corporation, ThoughtWire and Verto Health |
The global digital twins technologies market is estimated to be valued at USD 601.8 million in 2025.
The market size for the global digital twins technologies market is projected to reach approximately USD 1,771.3 million by 2035.
The global digital twins technologies market is expected to grow at a CAGR of 11.4% between 2025 and 2035.
The key types of digital twin formats in the global digital twins technologies market include Patient, Device, Facility, Process and others.
In terms of Application, Drug Discovery & Pre-clinical/Virtual trials is projected to command the highest share at 27.7% in the global digital twins technologies market in 2025.
Explore Similar Insights

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA