The Hernia Mesh Devices Market is estimated to be valued at USD 5.1 billion in 2025 and is projected to reach USD 7.1 billion by 2035, registering a compound annual growth rate (CAGR) of 3.4% over the forecast period.
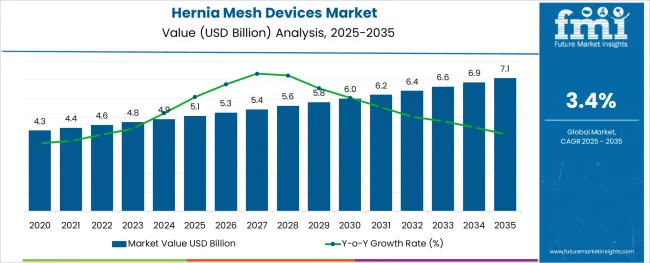
The hernia mesh devices market is expanding steadily, driven by the increasing prevalence of hernia cases worldwide and advancements in surgical repair techniques. Greater adoption of minimally invasive surgeries has increased the demand for reliable mesh implants that provide effective reinforcement and reduce recurrence rates.
Healthcare providers are focusing on improving patient outcomes by using materials that promote tissue integration and minimize complications. Growing awareness of inguinal hernia, one of the most common types, has further accelerated market growth.
Innovations in mesh design and biocompatibility have addressed concerns related to inflammation and infection. Additionally, the increasing number of hernia surgeries performed globally due to aging populations and rising obesity rates supports sustained demand. Future market opportunities are expected from the development of lightweight meshes and absorbable materials that improve recovery experiences. Segment growth is anticipated to be led by the Synthetic Mesh type and Inguinal Hernia as the leading hernia category.
The market is segmented by Mesh Type and Hernia Type and region. By Mesh Type, the market is divided into Synthetic Mesh and Biological Mesh. In terms of Hernia Type, the market is classified into Inguinal Hernia, Incisional Hernia, Femoral Hernia, and Others. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
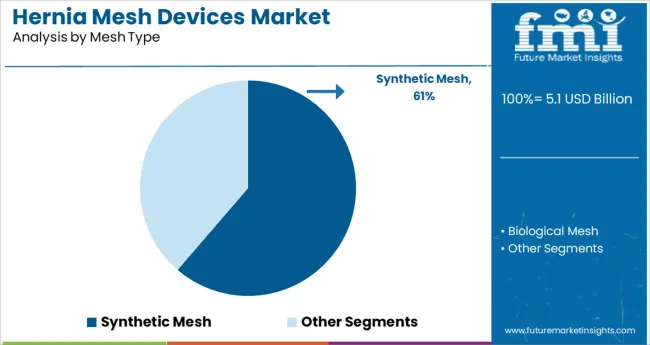
The Synthetic Mesh segment is projected to hold 61.3% of the hernia mesh devices market revenue in 2025, maintaining its leadership position. This segment’s growth is fueled by the widespread use of synthetic materials such as polypropylene and polyester, which offer durability and strength to support hernia repair.
These materials have been favored due to their ability to integrate well with host tissue and provide long-lasting reinforcement. Surgical teams prefer synthetic meshes for their availability in various configurations suited to different hernia types and surgical approaches.
Additionally, improvements in mesh coatings and manufacturing processes have reduced the risks of complications and enhanced biocompatibility. The synthetic mesh segment remains a cornerstone in hernia repair procedures because of its proven clinical effectiveness and versatility.
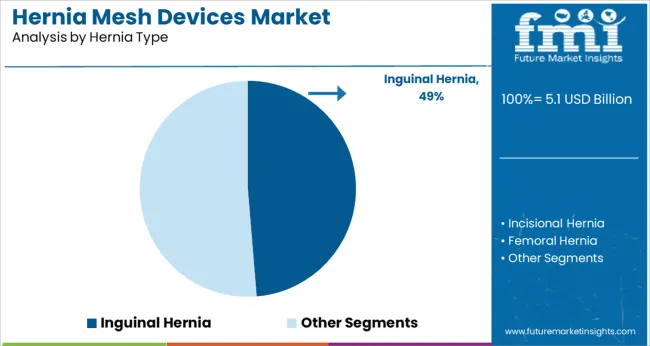
The Inguinal Hernia segment is expected to capture 48.7% of the market revenue in 2025, reflecting its position as the most common hernia type treated with mesh devices. This segment’s prominence is due to the high incidence of inguinal hernias in both males and females, especially in aging and physically active populations.
Clinical protocols recommend mesh implantation for inguinal hernia repair to reduce recurrence rates and improve postoperative recovery. The ease of surgical access and well-established repair techniques have made inguinal hernia the preferred focus area for mesh device applications.
Moreover, increasing awareness and screening programs have resulted in earlier diagnosis and treatment, driving consistent demand. As minimally invasive and laparoscopic procedures gain popularity, the use of mesh in inguinal hernia repairs is expected to grow steadily.
Global market for Hernia Mesh Devices expanded at a CAGR of 2.8% over the last six years (2020 to 2024). According to FDA, around 46% of the aged population has been observed to be the most active users of Hernia mesh devices. Nevertheless, buyers from other age groups will soon start purchasing them to make the demand more mainstream.
The global Hernia Mesh Devices Market is predicted to surge ahead at a CAGR of 3.4%. The United States will continue to be the largest consumer of Hernia Mesh Devices throughout the analysis period accounting for over USD 553.8 Million absolute dollar opportunity in the coming 10-year period.
According to a research published in the United Kingdom, the prevalence of hernias rises with age. The prevalence of hernia was found to be 5%, 10%, 18%, 24%, 31%, and 45% among the age groups of 25 - 34 years, 35 - 44 years, 45 - 54 years, 55 - 64 years, 65 - 74 years and 75 years and above, respectively.
The advancements such as self-fixating meshes and articulating fixation are achieved to overcome the issues, which makes it a key factor for the growth in the adoption of hernia mesh devices. The cost of raw material for hernia mesh though subjected to market volatility has never crossed USD 1,500 in any year providing a necessary profit margin to the manufacturer of hernia mesh devices.
Over 4.9 Million hernia operations take place worldwide annually. Non-degradable hernia surgical mesh implants are characterized by poor healing response, and in vivo erosion which leads to over 10% failure rate and an estimated 42% of recurrent hernias. In 4.921, the average global price of polypropylene was USD 1,285 per ton as compared to 4.94.9, while prices have increased roughly by 36% between these years.
This increase in the price of raw materials for making synthetic mesh adversely affected the market revenue of polypropylene hernia mesh devices.
The 3-D printed biological mesh minimized post-surgical complications of hernia repair and its raw material such as human or porcine dermis is widely available and hence not subjected to market volatility effectively making their price constant. The manufacturing of biological mesh is highly reproducible and scalable and it can be modified depending on the patient’s background.
The cost-utility of a biological mesh device with a 3-D printing technique for the treatment of hernia is not cost-effective for the hospital or surgeons who pay the retail price of the product but it is cost-effective from a third-party payer perspective like medical reimbursement by the government interventions. The cost of 3D printed biological mesh will be USD 1,813 which is cost-effective regardless of size, furthermore making it significantly lower than the current retail cost of synthetic mesh.
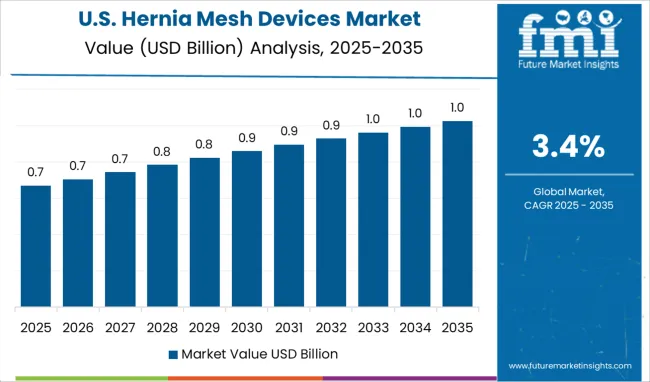
North America is anticipated to grow at 3.4% CAGR during the forecast period 2025 to 2035. In North America, the reimbursement policy for hernia mesh is favorable to the patients, which encourages them to choose laparoscopic surgery.
Patients have simple access to detailed payment codes and ratios that governments and industry players make public. In addition, technical improvements and the introduction and acceptance of new products, such as TELA Bio, are boosting the market forward.
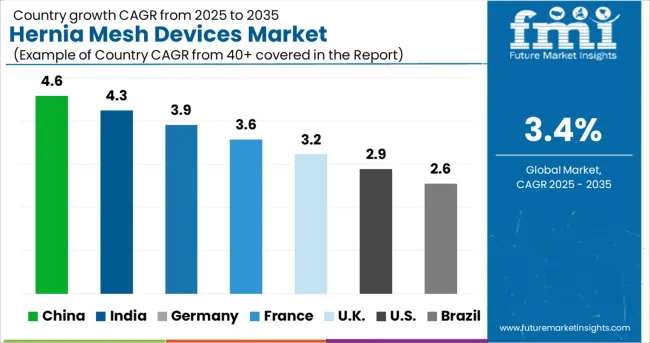
The United States will account for over 34% of the global Hernia Mesh Devices Market by the year 2035, representing it as a fertile ground for hernia mesh device manufacturers, especially synthetic mesh devices. Hernia Mesh Devices Market will grow a USD 2.9% CAGR between 2025 and 2035 for the United States.
The global annual death rate of hernia per 4.300 thousand individuals is 0.5, according to Health Grove. According to statistics by FDA, more than 4.3 Million hernia procedures happen annually in the United States out of which, about 800 thousand procedures are of the inguinal type, making it one of the largest market for Hernia Mesh Devices.
Hernia mesh devices revenue in China is expected to witness an absolute dollar opportunity of USD 5.4.3 Million during 2025 - 2035. With a projected CAGR of 2.6%, China will become a USD 7.4.3 Million Hernia Mesh Devices Market by the end of 2035.
It was observed that the number of tension-free hernia repair operations in China reached 4.3 Million in 204.35 with an increase of 20%-30% in such operations each year. In particular, an inguinal hernia is more prominent with a male-to-female ratio of 8.2 to 4.3.
According to China’s Hernia Registry, adult inguinal hernia also accounts for the largest proportion of 83.3%. A report by medical service information, 204.38, the cost of a single case of inguinal hernia was USD 922.6, indicating that cost control in county-wide hospitals was good, further creating a robust demand for hernia mesh devices in China.
Inguinal hernia constitutes the most revenue from sales of hernia mesh devices. Inguinal hernias account for over 80% of all hernias. It is also 10X more common in men than in women, thus necessitating the need for correction.
Hernia repair for primary and incisional hernias is the most common abdominal surgery performed all around the globe, according to the International Journal of Abdominal Wall and Hernia Surgery. Men have a 27-43% and women have a 3-6% lifetime risk of developing an inguinal hernia. It is expected to trigger the growth of hernia repair market.
Patient feasibility due to tension-free procedures and post-operative complications involved in classic surgical techniques are also contributing to the demand for Inguinal Hernia Mesh. In addition, the recurrence rate for inguinal hernia is 1-3%, which further imparts strain on the hernia mesh devices revenue.
A procedure of synthetic mesh for hernia repair surgery which ranges from USD 4,200 to USD 12,500 has hurt the capitalization of the Hernia Mesh Devices Market. Synthetic Mesh is used in around 90% of hernia surgeries according to FDA. In the Asia Pacific where 99% of Hernia surgery is performed with Synthetic Mesh, there is a significant growth opportunity for biological mesh for based hernia repair devices.
On the other hand, the biological mesh has yet to reach the majority of the consumers as it is still in the research process hence it captures only around 1% of the global Hernia Mesh Devices market.
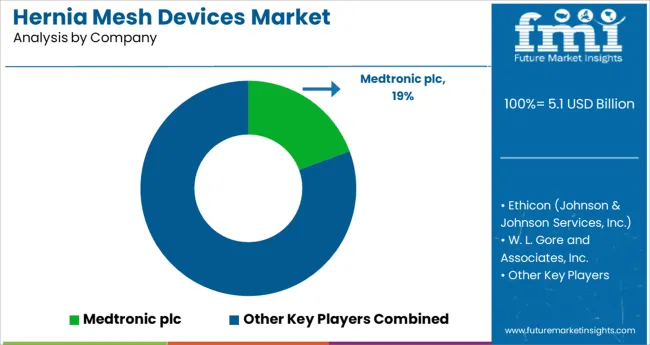
Most of the leading players in hernia mesh devices market have been focusing on obtaining approvals from regulatory authorities.
The key companies operating in the Hernia Mesh Devices market include Medtronic plc, Ethicon, and W. L. Gore and Associates, Inc., Atrium Medical Technologies, B. Braun Melsungen AG, PRIMEQUAL SA, Becton, Dickinson, and Company, Deep Blue Medical Inc., Dipromed Srl, BioCer Entwicklungs-GmbH, Betatech Medical, Sinolinks Medical Innovation, Inc, Aspide Medical S.A.S., Meril Life Sciences Pvt. Ltd., Herniamesh Srl., Changzhou Medical Equipment General Factory Co., Ltd, Gem Srl, SAMYANG HOLDING CORPORATION, Katsan Katgut Sanayi Ve Ticaret Anonim Sirketi, Novus Scientific AB.
Some of the recent developments by key providers of the Hernia Mesh Devices market are as follows:
Similarly, recent developments related to companies manufacturing hernia Mesh Devices have been tracked by the team at Future Market Insights, which is available in the full report.
| Attribute | Details |
|---|---|
| Forecast Period | 2025 to 2035 |
| Historical Data Available for | 2020 to 2024 |
| Market Analysis | million for Value |
| Key Countries Covered | USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, Russia, China, Japan, South Korea, India, Australia, South Africa, Saudi Arabia, UAE, and Israel. |
| Key Market Segments Covered | Mesh Type and Hernia Type |
| Key Companies Profiled | Medtronic plc; Ethicon; W. L. Gore and Associates, Inc; Atrium Medical Technologies; B. Braun Melsungen AG.; LifeCell Corporation; PRIMEQUAL SA; Becton, Dickinson, and Company; Deep Blue Medical Inc; Dipromed Srl; BioCer Entwicklungs-GmbH; Betatech Medical; Sinolinks Medical Innovation, Inc; Aspide Medical S.A.S.; Meril Life Sciences Pvt. Ltd; Herniamesh Srl; Changzhou Medical Equipment General Factory Co., Ltd; Gem Srl; SAMYANG HOLDING CORPORATION; Katsan Katgut Sanayi Ve Ticaret Anonim Sirketi; Novus Scientific AB |
| Report Coverage | Market Forecast, Competition Intelligence, DROT Analysis, Market Dynamics and Challenges, and Strategic Growth Initiatives |
| Customization & Pricing | Available upon Request |
The global hernia mesh devices market is estimated to be valued at USD 5.1 billion in 2025.
It is projected to reach USD 7.1 billion by 2035.
The market is expected to grow at a 3.4% CAGR between 2025 and 2035.
The key product types are synthetic mesh and biological mesh.
inguinal hernia segment is expected to dominate with a 48.7% industry share in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Hernia Protection Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Hernia Repair Devices Market Insights - Trends & Forecast 2025 to 2035
Ventral Hernia Treatment Market Size and Share Forecast Outlook 2025 to 2035
Mesh Bag Market Size and Share Forecast Outlook 2025 to 2035
Mesh Fabric Market Size and Share Forecast Outlook 2025 to 2035
Mesh Nebulizer for Kids Market Size and Share Forecast Outlook 2025 to 2035
Mesh Tarpaulin Sheets Market Size and Share Forecast Outlook 2025 to 2035
Understanding Market Share Trends in Mesh Bags
Foam Mesh Sleeves Market Growth - Demand & Forecast 2025 to 2035
Handheld Mesh Nebulizer Market Size and Share Forecast Outlook 2025 to 2035
Wireless Mesh Network Market Size and Share Forecast Outlook 2025 to 2035
Surgical Mesh Market
Monofilament Mesh Filter Bags Market Size and Share Forecast Outlook 2025 to 2035
Screen Printing Mesh Market Size and Share Forecast Outlook 2025 to 2035
Nylon Monofilament Mesh Filter Bags Market Size and Share Forecast Outlook 2025 to 2035
Breast Reconstruction Meshes Market Size and Share Forecast Outlook 2025 to 2035
Surgical Polypropylene Mesh Market
Biomaterial In Surgical Mesh Market Size and Share Forecast Outlook 2025 to 2035
FBAR Devices Market
Snare devices Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA