The instruments for peptide drug synthesis market enters a decade of expansion that will transform pharmaceutical research and biopharmaceutical production across academic institutions, biotechnology companies, and synthetic service organizations. The market's progression from USD 229.5 million in 2025 to USD 486.4 million by 2035 represents controlled growth, reflecting the accelerated adoption of automated synthesis technology and peptide therapeutics development across pharmaceutical manufacturing, research applications, and contract synthesis services worldwide.
The first half of the decade (2025 to 2030) will witness the market climbing from USD 229.5 million to approximately USD 335.0 million, adding USD 105.5 million in value, which constitutes 41% of the total forecast growth period. This phase will be characterized by the continued adoption of automated synthesis platforms over manual methods, driven by increasing pharmaceutical research demands and the growing need for efficient peptide production solutions in drug development and manufacturing applications globally. Enhanced automation capabilities and integrated monitoring systems will become standard expectations rather than premium options.

The latter half (2030 to 2035) will witness continued growth from USD 335.0 million to USD 486.4 million, representing an addition of USD 151.5 million or 59% of the decade's expansion. This period will be defined by mass market penetration of advanced synthesis platforms, integration with comprehensive pharmaceutical manufacturing systems, and seamless compatibility with existing laboratory infrastructure. The market trajectory signals fundamental shifts in how pharmaceutical companies approach peptide drug development and production operations, with participants positioned to benefit from growing demand across multiple application segments and synthesis channels.
Peptide synthesis instrumentation encompasses sophisticated automated systems coordinating solid-phase synthesis reactions, purification processes, and analytical monitoring across diverse pharmaceutical research and production applications. Modern synthesizers integrate multiple reaction chambers with automated amino acid coupling, deprotection cycles, and real-time monitoring capabilities enabling parallel synthesis of multiple peptide sequences. Synthesis scales typically range from research quantities of milligrams to production volumes exceeding kilograms, offering versatility for discovery research through commercial manufacturing applications.
Pharmaceutical and biotechnology companies drive primary instrument demand through requirements for drug discovery programs, lead optimization studies, and clinical supply manufacturing requiring precise control over synthesis parameters and product quality. Automated platforms provide enhanced reproducibility compared to manual synthesis methods while reducing labor requirements and synthesis cycle times. Contract synthesis organizations utilize high-throughput systems for client projects requiring rapid turnaround times and consistent quality standards across diverse peptide sequences.
Research institutions encompass academic laboratories, government research facilities, and hospital-based research programs requiring reliable synthesis capabilities for basic research, method development, and specialized applications. Educational applications include training programs for pharmaceutical chemistry and biotechnology students requiring hands-on experience with modern synthesis techniques and instrumentation.
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Peptide synthesizer systems | 37% | Automated platforms, research focus |
| Purification equipment | 35% | HPLC systems, chromatography | |
| Other instruments | 28% | Analytical equipment, accessories | |
| Pharmaceutical companies | 52% | Drug development, commercial production | |
| Synthetic services | 28% | Contract manufacturing, specialized synthesis | |
| Research institutions | 20% | Academic research, method development | |
| Future (3-5 yrs) | Advanced synthesizer platforms | 40-45% | Technology integration, automation gains |
| Integrated purification systems | 32-38% | Process optimization, workflow enhancement | |
| Enhanced analytical instruments | 22-28% | Quality control, method development | |
| Expanded pharmaceutical applications | 50-55% | Pipeline growth, production scaling | |
| Enhanced synthetic services | 25-30% | Outsourcing expansion, specialized capabilities | |
| Advanced research platforms | 18-22% | Technology adoption, capability enhancement | |
| Digital integration platforms | 5-8% | IoT connectivity, data analytics |
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) | USD 229.5 million |
| Market Forecast (2035) | USD 486.4 million |
| Growth Rate | 7.8% CAGR |
| Leading Product | Peptide Synthesizer Segment |
| Primary Application | Pharmaceutical Companies |
The market demonstrates strong fundamentals with peptide synthesizer systems capturing a dominant share through reliable automation capabilities and synthesis optimization. Pharmaceutical company applications drive primary demand, supported by increasing drug development requirements and operational efficiency development. Geographic expansion remains concentrated in developed markets with established pharmaceutical infrastructure, while emerging economies show accelerating adoption rates driven by research modernization initiatives and rising biotechnology investment standards.
Primary Classification: The market segments by product into peptide synthesizer (37%) and purification equipment (35%) systems, representing the evolution from manual synthesis methods to integrated automated platforms for comprehensive pharmaceutical research and production optimization.
Secondary Classification: Application segmentation divides the market into pharmaceutical and biotechnology companies (52%), synthetic services company (28%), and research institutions (20%), reflecting distinct requirements for synthesis capacity, automation efficiency, and research infrastructure standards.
The segmentation structure reveals technology progression from standard synthesis methods toward specialized instrument applications with enhanced operational consistency and automation capabilities, while application diversity spans from pharmaceutical development to research applications requiring precise synthesis solutions.

Market Position: Peptide synthesizer systems command the leading position in the synthesis instrument market with 37% market share through advanced automation features, including superior synthesis capabilities, operational reliability, and process optimization that enable laboratories to achieve optimal peptide production across diverse research and manufacturing applications.
Value Drivers: The segment benefits from laboratory preference for automated synthesis systems that provide consistent performance characteristics, reduced manual complexity, and operational efficiency optimization without requiring significant infrastructure modifications. Advanced design features enable automated reaction monitoring systems, yield optimization, and integration with existing laboratory equipment, where synthesis performance and reproducibility represent critical user requirements.
Competitive Advantages: Peptide synthesizer systems differentiate through proven operational reliability, consistent synthesis characteristics, and integration with automated laboratory management systems that enhance production effectiveness while maintaining optimal quality standards for diverse pharmaceutical and research applications.
Key market characteristics:
Purification equipment maintains a 35% market position in the synthesis instrument market due to enhanced separation properties and downstream processing characteristics. These systems appeal to laboratories requiring specialized performance with premium positioning for pharmaceutical and biotechnology purification applications. Market growth is driven by quality requirements expansion, emphasizing integrated purification solutions and operational efficiency through optimized equipment designs.
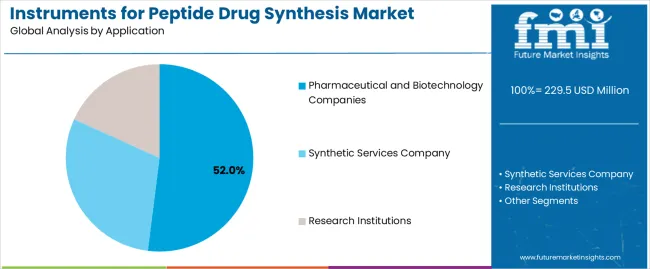
Market Context: Pharmaceutical and biotechnology company applications demonstrate market leadership in the peptide synthesis instrument market with 52% share due to widespread adoption of automated synthesis systems and increasing focus on drug development efficiency, research productivity, and applications that maximize synthesis capacity while maintaining quality standards.
Appeal Factors: Pharmaceutical companies prioritize synthesis reliability, operational efficiency, and integration with existing research infrastructure that enables coordinated drug development operations across multiple synthesis applications. The segment benefits from substantial research investment and modernization programs that emphasize the acquisition of automated systems for development optimization and synthesis efficiency applications.
Growth Drivers: Pharmaceutical development expansion programs incorporate peptide synthesis instruments as standard equipment for research operations, while drug pipeline growth increases demand for consistent synthesis capabilities that comply with quality standards and minimize development complexity.
Application dynamics include:
Synthetic services company applications capture 28% market share through comprehensive synthesis requirements in contract manufacturing, specialized synthesis operations, and client service applications. These operations demand reliable synthesis systems capable of handling diverse projects while providing effective capacity management and operational performance capabilities.
Research institution applications account for 20% market share, including academic laboratories, government research facilities, and educational applications requiring specialized synthesis solutions for research optimization and method development capabilities.
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Pharmaceutical pipeline growth & peptide drug development (research expansion, therapeutic development) | ★★★★★ | Large-scale pharmaceutical markets require efficient, reliable synthesis solutions with consistent performance and quality compliance across development applications. |
| Driver | Automation adoption & efficiency optimization (labor reduction, process standardization) | ★★★★★ | Drives demand for automated synthesis solutions and high-capacity production capabilities; providers offering integrated platforms gain competitive advantage. |
| Driver | Contract synthesis expansion & outsourcing trends (service growth, specialized capabilities) | ★★★★☆ | Research organizations need specialized synthesis solutions; demand for contract services expanding addressable market segments. |
| Restraint | High instrument costs & capital requirements (equipment complexity, maintenance needs) | ★★★★☆ | Small laboratories face capital pressure; increases cost sensitivity and affects equipment availability in budget-sensitive markets. |
| Restraint | Technical complexity & specialized expertise (operator training, method development) | ★★★☆☆ | Applications require specialized knowledge and technical support, limiting adoption in resource-constrained research environments. |
| Trend | Digital integration & data analytics (connected instruments, process monitoring) | ★★★★★ | Growing demand for smart laboratory equipment; digital integration becomes core value proposition in modern research environments. |
| Trend | Asian market expansion & research investment (regional development, biotechnology growth) | ★★★★☆ | Regional research expansion drives demand for synthesis equipment; local capabilities drive competition toward technology advancement. |
The instruments for peptide drug synthesis market demonstrates varied regional dynamics with growth leaders including China (10.5% growth rate) and India (9.8% growth rate) driving expansion through research initiatives and pharmaceutical development. Steady Performers encompass Germany (9.0% growth rate), Brazil (8.2% growth rate), and USA (7.4% growth rate), benefiting from established pharmaceutical industries and advanced research adoption. Mature Markets feature UK (6.6% growth rate) and Japan (5.9% growth rate), where instrument technology advancement and quality standardization requirements support consistent growth patterns.
Regional synthesis reveals East Asian and South American markets leading adoption through pharmaceutical expansion and research development, while Western countries maintain steady expansion supported by technology advancement and regulatory standardization requirements. Emerging markets show strong growth driven by biotechnology applications and research modernization trends.

| Region/Country | 2025 to 2035 Growth | How to win | What to watch out |
|---|---|---|---|
| China | 10.5% | Focus on cost-effective synthesis solutions | Infrastructure challenges; equipment availability |
| India | 9.8% | Lead with high-capacity research systems | Regulatory requirements; technical complexity |
| Germany | 9.0% | Provide quality-compliant applications | Over-specification; regulatory compliance |
| Brazil | 8.2% | Offer comprehensive research services | Market maturity; equipment costs |
| USA | 7.4% | Premium quality positioning | Technology saturation; capital costs |
| UK | 6.6% | Push pharmaceutical integration solutions | Equipment precision; market maturity |
| Japan | 5.9% | Premium efficiency positioning | Equipment precision; market maturity |

China establishes fastest market growth through aggressive pharmaceutical programs and comprehensive research capacity development, integrating advanced peptide synthesis instruments as standard components in biotechnology facilities and pharmaceutical research installations. The country's 10.5% growth rate reflects government initiatives promoting biotechnology modernization and pharmaceutical development capabilities that mandate the use of automated synthesis systems in research facilities. Growth concentrates in major pharmaceutical centers, including Beijing, Shanghai, and Guangzhou, where research technology development showcases integrated synthesis systems that appeal to companies seeking advanced production optimization capabilities and operational pharmaceutical applications.
Chinese instrument distributors are developing cost-effective synthesis solutions that combine domestic manufacturing advantages with advanced operational features, including automated monitoring systems and enhanced reliability capabilities. Distribution channels through pharmaceutical suppliers and research equipment distributors expand market access, while government support for biotechnology development supports adoption across diverse research segments.
Strategic Market Indicators:
In Karnataka, Maharashtra, and Delhi regions, pharmaceutical facilities and research operations are implementing advanced peptide synthesis instruments as standard equipment for research optimization and operational development enhancement, driven by increasing government pharmaceutical investment and biotechnology modernization programs that emphasize the importance of synthesis capabilities. The market holds a 9.8% growth rate, supported by government research initiatives and pharmaceutical development programs that promote advanced synthesis systems for research facilities.
Indian operators are adopting synthesis systems that provide consistent operational performance and quality compliance features, appealing in pharmaceutical regions where research efficiency and quality standards represent critical operational requirements. Market expansion benefits from growing pharmaceutical processing capabilities and research integration agreements that enable domestic production of synthesis equipment for biotechnology applications.
Germany establishes quality-compliant market development through comprehensive pharmaceutical programs and established research infrastructure, integrating peptide synthesis instruments across pharmaceutical facilities and biotechnology applications. The country's 9.0% growth rate reflects mature pharmaceutical industry relationships and established equipment adoption that supports widespread use of synthesis systems in research facilities and quality-compliant operations. Growth concentrates in major pharmaceutical centers, including North Rhine-Westphalia, Bavaria, and Baden-Württemberg, where research technology showcases mature instrument deployment that appeals to companies seeking proven quality capabilities and operational efficiency applications.
German instrument distributors leverage established distribution networks and comprehensive quality capabilities, including compliance programs and technical support that create customer relationships and operational advantages. The market benefits from mature quality standards and regulatory requirements that support synthesis system use while supporting technology advancement and operational optimization.
Brazil establishes research service development through expanding pharmaceutical programs and growing research infrastructure, integrating peptide synthesis systems across biotechnology facilities and pharmaceutical applications. The country's 8.2% growth rate reflects growing pharmaceutical industry relationships and increasing equipment adoption that supports expanding use of synthesis systems in Brazilian research facilities. Growth concentrates in major pharmaceutical areas, including São Paulo, Rio de Janeiro, and Minas Gerais, where research technology development showcases integrated synthesis systems that appeal to Brazilian companies seeking advanced research solutions with operational efficiency compatibility.
Brazilian instrument distributors focus on maintaining service standards while adopting research synthesis efficiency, creating demand for systems that balance performance with operational advantages. The market benefits from strong pharmaceutical infrastructure and growing biotechnology opportunities that support synthesis technology adoption while maintaining quality standards important to Brazilian pharmaceutical applications.
USA's advanced pharmaceutical technology market demonstrates sophisticated peptide synthesis integration with documented operational effectiveness in premium research applications and modern facility installations through integration with existing quality systems and pharmaceutical infrastructure. The country maintains a 7.4% growth rate, leveraging traditional quality expertise and precision systems integration in synthesis technology. Pharmaceutical centers, including California, Massachusetts, and New Jersey, showcase premium installations where synthesis systems integrate with traditional quality platforms and modern facility management systems to optimize research operations and maintain pharmaceutical quality profiles.
American instrument distributors prioritize equipment precision and quality consistency in synthesis development, creating demand for premium systems with advanced features, including quality monitoring and automated synthesis systems. The market benefits from established quality infrastructure and commitment to pharmaceutical standards that provide long-term operational benefits and compliance with traditional quality research methods.
UK establishes pharmaceutical integration development through comprehensive research programs and established pharmaceutical infrastructure, integrating peptide synthesis systems across biotechnology facilities and specialized pharmaceutical applications. The country's 6.6% growth rate reflects growing pharmaceutical investment and increasing adoption of synthesis technology that supports expanding use of automated systems in British pharmaceutical facilities. Growth concentrates in major pharmaceutical areas, including Cambridge, Oxford, and London, where research technology development showcases integrated synthesis systems that appeal to British companies seeking advanced pharmaceutical solutions with research efficiency compatibility.
British instrument distributors focus on maintaining quality standards while adopting pharmaceutical synthesis efficiency, creating demand for systems that balance performance with operational advantages. The market benefits from strong pharmaceutical infrastructure and growing biotechnology opportunities that support synthesis technology adoption while maintaining quality standards important to British pharmaceutical applications.
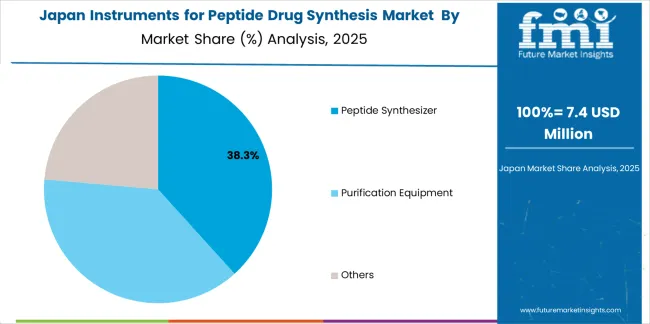
Japan's advanced pharmaceutical technology market demonstrates sophisticated peptide synthesis integration with documented operational effectiveness in premium research applications and modern facility installations through integration with existing efficiency systems and pharmaceutical infrastructure. The country maintains a 5.9% growth rate, leveraging traditional efficiency expertise and precision systems integration in synthesis technology. Pharmaceutical centers, including Tokyo, Osaka, and Kanagawa, showcase premium installations where synthesis systems integrate with traditional efficiency platforms and modern facility management systems to optimize research operations and maintain pharmaceutical efficiency profiles.
Japanese instrument distributors prioritize equipment precision and efficiency consistency in synthesis development, creating demand for premium systems with advanced features, including efficiency monitoring and automated synthesis systems. The market benefits from established efficiency infrastructure and commitment to pharmaceutical standards that provide long-term operational benefits and compliance with traditional quality research methods.
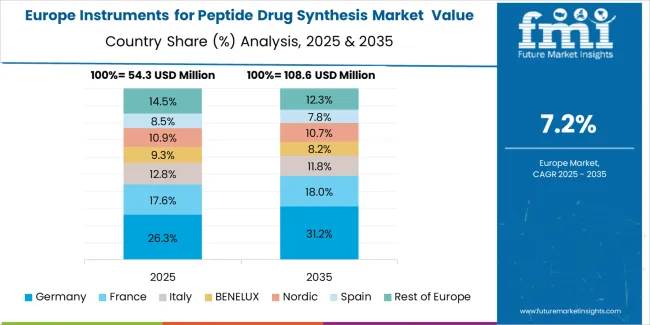
The European instruments for peptide drug synthesis market is projected to represent a significant portion of global consumption, with strong regional distribution across major economies. Germany is expected to maintain its leadership position with USD 78.1 million in 2025, accounting for substantial European market share, supported by its advanced pharmaceutical infrastructure and major research centers.
United Kingdom follows with USD 7.9 million, representing significant European market share in 2025, driven by comprehensive pharmaceutical programs and research technology development initiatives. France holds USD 12.4 million through specialized biotechnology applications and pharmaceutical compliance requirements. Italy commands USD 157.7 million, while Spain accounts for USD 170.0 million in 2025. The rest of Europe region maintains USD 183.2 million, attributed to increasing synthesis system adoption in Nordic countries and emerging pharmaceutical facilities implementing research modernization programs.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global platforms | Instrument networks, broad product portfolios, manufacturing facilities | Proven reliability, multi-region support, comprehensive service | Technology refresh cycles; customer lock-in dependency |
| Technology innovators | R&D capabilities; advanced synthesis systems; digital interfaces | Latest technology first; attractive ROI on specialized applications | Service density outside core regions; customization complexity |
| Regional specialists | Local sourcing, fast delivery, nearby technical support | "Close to site" support; pragmatic pricing; local regulations | Technology gaps; talent retention in engineering |
| Application-focused ecosystems | Industry expertise, technical support, specialized solutions | Lowest application variation; comprehensive industry support | Scaling costs if overpromised; technology obsolescence |
| Service specialists | Maintenance programs, equipment supply, technical training | Win service-intensive applications; flexible support | Scalability limitations; narrow market focus |
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 229.5 million |
| Product | Peptide Synthesizer, Purification Equipment, Others |
| Application | Pharmaceutical and Biotechnology Companies, Synthetic Services Company, Research Institutions |
| Regions Covered | East Asia, Western Europe, South Asia Pacific, North America, Latin America, Middle East & Africa |
| Countries Covered | China, Germany, United States, Japan, India, Brazil, United Kingdom, and 25+ additional countries |
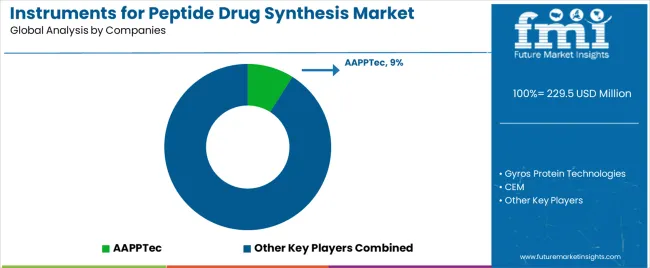
| Key Companies Profiled | AAPPTec, Gyros Protein Technologies, CEM, Shimadzu, Biotage, Peptide Scientific, CSBio, Activotec, Hainan JBPharm |
| Additional Attributes | Dollar sales by product and application categories, regional adoption trends across East Asia, Western Europe, and South Asia Pacific, competitive landscape with instrument manufacturers and service providers, user preferences for synthesis consistency and operational reliability, integration with pharmaceutical platforms and laboratory monitoring systems, innovations in synthesis technology and instrument enhancement, and development of advanced peptide synthesis solutions with enhanced performance and research optimization capabilities. |
The global instruments for peptide drug synthesis market is estimated to be valued at USD 229.5 million in 2025.
The market size for the instruments for peptide drug synthesis market is projected to reach USD 486.4 million by 2035.
The instruments for peptide drug synthesis market is expected to grow at a 7.8% CAGR between 2025 and 2035.
The key product types in instruments for peptide drug synthesis market are peptide synthesizer, purification equipment and others.
In terms of application, pharmaceutical and biotechnology companies segment to command 52.0% share in the instruments for peptide drug synthesis market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
qPCR Instruments Market Analysis - Growth, Trends & Forecast 2025 to 2035
Modular Instruments Market Growth – Trends & Forecast 2025 to 2035
Surgical Instruments Tracking System Market Growth - Trends & Forecast 2025 to 2035
Surgical Instruments Packaging Market Size, Share & Forecast 2025 to 2035
Digital Writing Instruments Market Size and Share Forecast Outlook 2025 to 2035
Surgical Suction Instruments Market
Hazardous Weighing Instruments Market Size, Growth, and Forecast for 2025 to 2035
Electronic Musical Instruments Market Size and Share Forecast Outlook 2025 to 2035
Microplate Handling Instruments Market Size and Share Forecast Outlook 2025 to 2035
Reusable Laparoscopic Instruments Market is segmented by Reusable Laparoscopic Scissors and Reusable Hand Instruments from 2025 to 2035
Handheld Arthroscopic Instruments Market Growth – Trends & Forecast 2025 to 2035
Disposable Laparoscopic Instruments Market Analysis – Size, Share & Forecast 2025-2035
Industrial Dust Detector Instruments Market Size and Share Forecast Outlook 2025 to 2035
Life Science and Chemical Instruments Market Analysis - Growth & Forecast 2024 to 2034
Coating Thickness Measurement Instruments Market
General Purpose Electronic Test and Measurement Instruments Market Analysis and Forecast by Product, End Use, and Region Through 2035
Formaldehyde Removal Air Purifier Market Size and Share Forecast Outlook 2025 to 2035
Fortified Dairy Products Market Size and Share Forecast Outlook 2025 to 2035
Form-Fill-Seal (FFS) Films Market Size and Share Forecast Outlook 2025 to 2035
Formable Films Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA