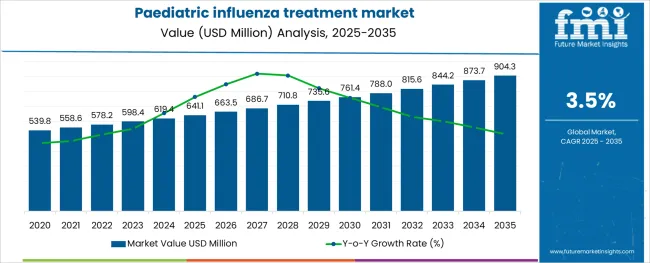
The Paediatric influenza treatment market is estimated to be valued at USD 641.1 million in 2025 and is projected to reach USD 904.3 million by 2035, registering a compound annual growth rate (CAGR) of 3.5% over the forecast period.
The paediatric influenza treatment market is growing steadily due to the increasing incidence of influenza infections among children worldwide. Healthcare providers are prioritizing early intervention to reduce complications and hospitalizations. The rising awareness of influenza severity in young populations and the availability of targeted therapies have supported wider treatment adoption.
Hospitals remain the primary care setting for managing paediatric influenza cases, especially severe or complicated infections. Advances in diagnostic capabilities have enabled timely identification and treatment, which has improved patient outcomes.
Increased government initiatives and vaccination programs aimed at reducing influenza burden in children have also helped expand treatment markets. Moving forward, innovations in antiviral therapies and improved healthcare infrastructure are expected to support continued growth. The market’s expansion is anticipated to be led by neuraminidase inhibitors as the primary treatment type and hospitals as the main end users.
The market is segmented by Treatment Type and End User and region. By Treatment Type, the market is divided into Neuraminidase Inhibitors, Adamantanes, Amantadine & Rimantadine, and Oseltamivir & Zanamivir. In terms of End User, the market is classified into Hospitals and Clinics.
Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
The market is segmented by Treatment Type and End User and region. By Treatment Type, the market is divided into Neuraminidase Inhibitors, Adamantanes, Amantadine & Rimantadine, and Oseltamivir & Zanamivir. In terms of End User, the market is classified into Hospitals and Clinics.
Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
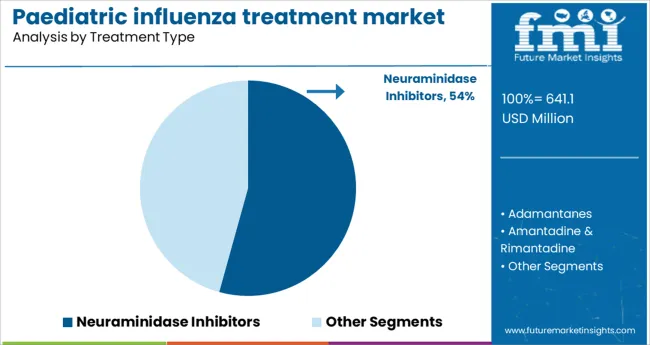
The neuraminidase inhibitors segment is projected to hold 54.3% of the paediatric influenza treatment market revenue in 2025. This segment’s growth has been driven by the widespread clinical use of neuraminidase inhibitors to reduce the duration and severity of influenza symptoms. These antivirals are favored due to their effectiveness against common influenza strains and their role in preventing complications such as secondary bacterial infections.
Their established safety profile in children has encouraged prescribing practices across pediatric healthcare settings. Additionally, the segment benefits from ongoing research supporting early administration post-infection onset.
The ability to manage both seasonal and pandemic influenza outbreaks with these inhibitors has further reinforced their prominence in treatment protocols.
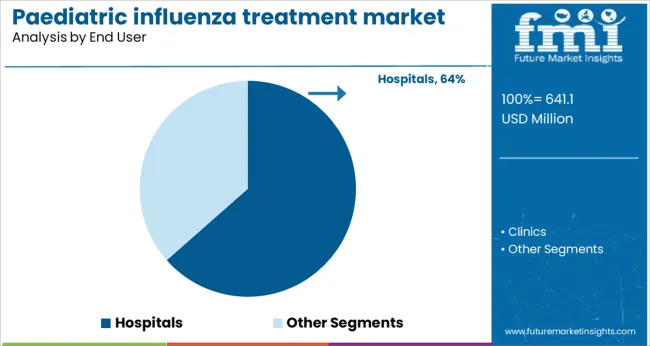
The hospitals segment is expected to account for 63.5% of the paediatric influenza treatment market revenue in 2025, maintaining its dominance among end users. This is due to the critical role hospitals play in treating severe influenza cases and managing outbreaks, especially in pediatric wards.
Hospitals provide comprehensive care including antiviral therapy, monitoring for complications, and supportive treatment. Their capacity to diagnose and treat influenza promptly helps reduce morbidity and healthcare costs associated with prolonged illness.
Moreover, hospitals often serve as centers for vaccination campaigns and public health education, which indirectly supports treatment uptake. The expanding pediatric patient population and increased hospitalization rates during peak influenza seasons have contributed to sustained demand in this segment.
Antiviral prescriptions are viable for the counteractive action of flu which is utilized for treatment and can diminish the span and seriousness of the disease and are likely to grow the paediatric influenza treatment market future trends in the coming year.
The development of the four authorized antiviral specialists, including oseltamivir, zanamivir, amantadine, and rimantadine, are some flowing flu infection strains that are likely to rise in the paediatric influenza treatment market analysis during the forecast period.
Expanding indications of flu between the age group of 1 to 9 years, coupled with a rise in the usage of antiviral drugs, have significantly contributed to enlarging the paediatric influenza treatment market growth.
The three influenza antiviral medicines approved by the USA (FDA) prescribed which are used during the flu season are oral oseltamivir (accessible as a generic drug or under the exchange name Tamiflu), zanamivir (Relenza - trade name), and intravenous peramivir (trade name Rapivab).
There is raising awareness of the adoption of paediatric influenza treatment.
Late innovative headways in restorative antiviral drugs for treating different types of this sickness have additionally caused an expanded request worldwide, which will keep on contributing towards the development of paediatric influenza treatment market opportunities in the coming period.
However, there are factors such as a limited number of treatment options in developing economies coupled with the high very high cost of branded drugs, expensiveness, and inefficient usage of low-cost non-prescribed drugs, less availability of antiviral drugs with improper guidelines are the key factors that may restraint the paediatric influenza treatment market growth.
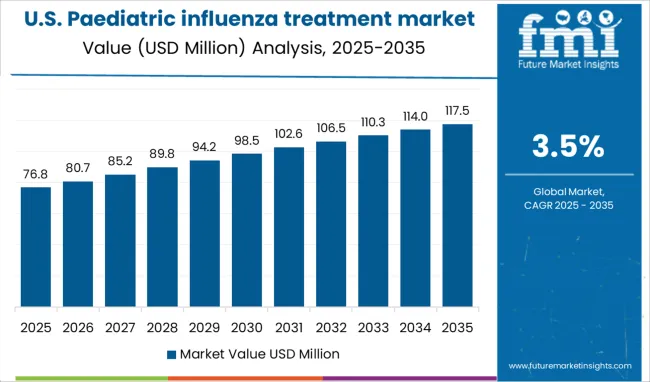
The North American region is expected to dominate the paediatric influenza treatment market share in comparison to other regions. The region acquires 34.0% of the share in the paediatric influenza treatment market size all around the region during the forecast period from 2025 to 2035.
The higher infection rate among children, higher cost of antiviral drugs and large population of patients of young age (1-9 years) suffering from paediatric influenza are leading to influence the paediatric influenza treatment market trends & forecast during the forthcoming year.
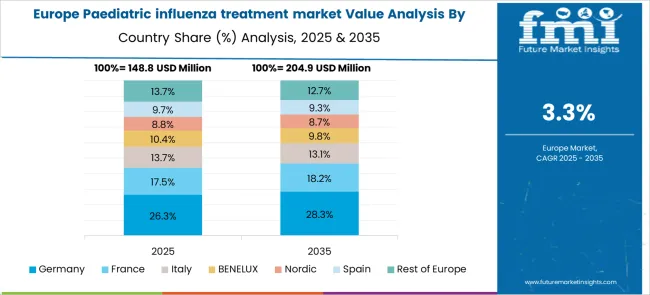
The Europe region is anticipated to grow the paediatric influenza treatment market share during the forecast period from 2025 to 2035 with a 27.3% of share in the paediatric influenza treatment market size.
High awareness levels among physicians and frequent publication of results of upcoming treatments tend to create a positive sentiment in the region, which is likely to rise the paediatric influenza treatment market demand analysis.
Healthy as well as chronically both may ill children due to the paediatric influenza virus itself or secondary bacterial infection are anticipated to grow the paediatric influenza treatment market growth.
Children should be treated and tested with neuraminidase inhibitors treatments which are more effective and tolerated against the virus and are supposed to grow the paediatric influenza treatment market future trends during the forecast period from 2025 to 2035.
Seasonal paediatric influenza treatment vaccine is more effective that helps to prevent and control the virus outbreaks.
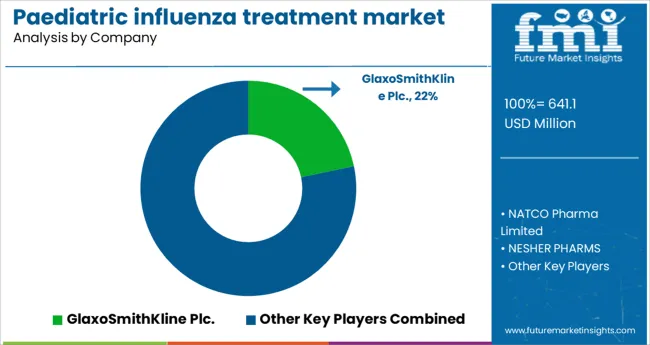
A few key players in the paediatric influenza treatment market are GlaxoSmithKline Plc., NATCO Pharma Limited., NESHER PHARMS, F. Hoffmann-La Roche Ltd, Atabay Kimya Sanayi ve Ticaret A.S., Cipla, Hetero Pharma, Teva Pharmaceutical Pvt. Ltd. and Olainfarm JSC.
The key player industries are emerging trends in paediatric influenza treatment market by adopting various market growth strategies including collaborations, mergers, partnerships, acquisitions, product launches and others.
Some of the recent developments in the paediatric influenza treatment market are as follows:
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 3.5% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in USD Million and CAGR from 2025-2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered | Treatment Type, End User, Region |
| Regions Covered | North America; Latin America; Europe; East Asia; South Asia; Oceania; Middle East and Africa |
| Key Countries Profiled | USA, Canada, Brazil, Argentina, Germany, UK, France, Spain, Italy, Nordics, BENELUX, Australia & New Zealand, China, India, ASEAN, GCC, South Africa |
| Key Companies Profiled | GlaxoSmithKline Plc; NATCO Pharma Limited; NESHER PHARMS; F. Hoffmann-La Roche Ltd; Atabay Kimya Sanayi ve Ticaret A.S.; Cipla; Hetero Pharma; Teva Pharmaceutical pvt. Ltd; Olainfarm JSC |
| Customization | Available Upon Request |
The global paediatric influenza treatment market is estimated to be valued at USD 641.1 million in 2025.
It is projected to reach USD 904.3 million by 2035.
The market is expected to grow at a 3.5% CAGR between 2025 and 2035.
The key product types are neuraminidase inhibitors, adamantanes, amantadine & rimantadine and oseltamivir & zanamivir.
hospitals segment is expected to dominate with a 63.5% industry share in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Paediatric Vaccine Market Size and Share Forecast Outlook 2025 to 2035
Paediatric Wheelchairs Market Size and Share Forecast Outlook 2025 to 2035
Paediatric Sports Medicine Market Size and Share Forecast Outlook 2025 to 2035
Paediatric Oncology Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Paediatric & Neonatal Testing Kits Market – Growth & Forecast 2025 to 2035
Paediatric Respiratory Disease Therapeutics Market
Paediatric Respiratory Syncytial Virus Infection Market Growth - Trends & Forecast 2025 to 2035
Paediatric Spasticity Treatment Market Size and Share Forecast Outlook 2025 to 2035
Paediatric Neuropsychiatric Disorders Treatment Market
Influenza Antiviral Market
Influenza Home Testing Market
Avian Influenza Antigen Testing Market Size and Share Forecast Outlook 2025 to 2035
Seasonal Influenza Vaccines Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
Marketing Mix Optimisation Market Size and Share Forecast Outlook 2025 to 2035
Marketing Sample Kits Market Size and Share Forecast Outlook 2025 to 2035
Marketing Transcription Market by Solution, Type, Industry, and Region – Growth, Trends, and Forecast through 2025 to 2035
Marketing Calendar Software Market Insights - Trends & Forecast 2025 to 2035
Geomarketing Market Size and Share Forecast Outlook 2025 to 2035
AI Marketing Tool Market Analysis – Growth & Future Demand 2024-2034
Hob Market Forecast and Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA