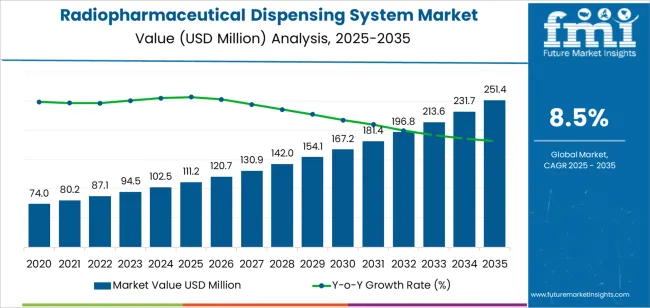
The global radiopharmaceutical dispensing system market is projected to reach USD 251.4 million by 2035, recording an absolute increase of USD 140.2 million over the forecast period. The market is valued at USD 111.2 million in 2025 and is expected to grow at a CAGR of 8.5% over the assessment period. The overall market size is expected to grow by nearly 2.3 times during the same period, supported by increasing demand for nuclear medicine diagnostic and therapeutic procedures worldwide, driving demand for automated radiopharmaceutical handling solutions and rising investments in radiopharmacy infrastructure and radiation safety enhancement programs globally. Complex regulatory requirements and substantial capital investment needs may pose challenges to market expansion.
The expansion trajectory reflects fundamental shifts in nuclear medicine delivery models, where centralized radiopharmaceutical preparation has emerged as a critical strategy for optimizing workflow efficiency and radiation protection in diagnostic imaging and therapeutic applications. Healthcare systems face mounting pressure to implement comprehensive dispensing solutions that integrate dose preparation, quality control verification, and distribution logistics while minimizing radiation exposure to pharmacy personnel and transportation staff.
Advanced dispensing systems deliver complete workflow automation from bulk radiopharmaceutical receipt through unit-dose preparation and delivery documentation, enabling nuclear medicine departments to achieve operational efficiency improvements of 40-60% while reducing cumulative staff radiation exposure through elimination of manual handling during routine dose subdivision and packaging procedures.
Regional market dynamics show pronounced variation, with developed markets emphasizing comprehensive automation integration and regulatory compliance enhancement while emerging economies prioritize foundational radiopharmacy infrastructure establishment and nuclear medicine service expansion. Centralized radiopharmacies and hospital nuclear medicine departments represent the primary demand centers, where fully automatic and semi-automatic dispensing systems integrate with hot cell configurations to standardize preparation workflows and contamination control measures. The technology landscape encompasses various automation levels and throughput capacities, creating diverse product offerings tailored to specific facility operational scales and service coverage requirements across pharmaceutical manufacturing, hospital, and research applications.
Investment patterns indicate sustained commitment from healthcare organizations and radiopharmaceutical manufacturers toward dispensing infrastructure that supports quality standardization and personnel safety enhancement objectives. Radiopharmacy directors pursue systems that consolidate multiple preparation steps while improving traceability and documentation, driving adoption of integrated automation platforms despite substantial implementation costs. The convergence of nuclear medicine utilization growth, theranostic therapy emergence, and occupational safety prioritization establishes favorable conditions for market expansion through 2035, though implementation challenges related to validation complexity and facility modification requirements will require ongoing attention from manufacturers and healthcare operators to ensure successful system integration across diverse radiopharmacy environments and operational configurations.
Between 2025 and 2030, the radiopharmaceutical dispensing system market is projected to expand from USD 111.2 million to USD 167.2 million, resulting in a value increase of USD 56 million, which represents 39.9% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for centralized radiopharmacy models and automated dose preparation solutions in nuclear medicine facilities, product innovation in robotic handling systems and integrated quality control technologies, as well as expanding integration with radiopharmacy information management systems and electronic health record platforms. Companies are establishing competitive positions through investment in advanced automation technologies, radiation shielding innovations, and strategic market expansion across pharmaceutical, hospital, research, and other nuclear medicine applications.
From 2030 to 2035, the market is forecast to grow from USD 167.2 million to USD 251.4 million, adding another USD 84.2 million, which constitutes 60.1% of the overall ten-year expansion. This period is expected to be characterized by the expansion of specialized dispensing systems, including advanced fully automatic configurations and integrated radiopharmacy solutions tailored for specific therapeutic radionuclide requirements, strategic collaborations between system manufacturers and radiopharmaceutical developers, and an enhanced focus on workflow optimization and regulatory compliance automation. The growing emphasis on alpha-emitter therapy and personalized nuclear medicine will drive demand for advanced, high-performance radiopharmaceutical dispensing solutions across diverse healthcare applications.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 111.2 million |
| Market Forecast Value (2035) | USD 251.4 million |
| Forecast CAGR (2025-2035) | 8.5% |
The radiopharmaceutical dispensing system market grows by enabling nuclear medicine facilities to achieve comprehensive workflow automation and radiation safety optimization while supporting increasing procedural volumes. Radiopharmacy operations face mounting pressure to implement integrated dispensing solutions that streamline preparation processes, with automated systems typically reducing dose preparation time by 50-70% compared to manual methods while maintaining pharmaceutical quality standards, making comprehensive automation essential for high-volume facilities serving multiple clinical departments. The centralization trend in radiopharmacy services creates demand for sophisticated dispensing platforms that can manage complex distribution logistics across satellite nuclear medicine departments while ensuring dose accuracy and delivery timeliness.
Government initiatives promoting radiation protection standards and pharmaceutical quality assurance frameworks drive adoption in pharmaceutical, hospital, research, and other applications, where dispensing automation has a direct impact on operational efficiency and regulatory compliance. The global expansion of nuclear medicine services and radiopharmaceutical therapy accelerates system demand as healthcare organizations seek comprehensive solutions capable of handling diverse radionuclides including diagnostic tracers, therapeutic isotopes, and emerging alpha-emitters while maintaining sterility and contamination control throughout preparation workflows. Substantial system acquisition costs and facility infrastructure modification requirements may limit adoption rates among smaller nuclear medicine programs and regions with developing radiopharmacy capabilities.
The market is segmented by automation level, application, and region. By automation level, the market is divided into fully automatic and semi-automatic. Based on application, the market is categorized into pharmaceutical, hospital, and research. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
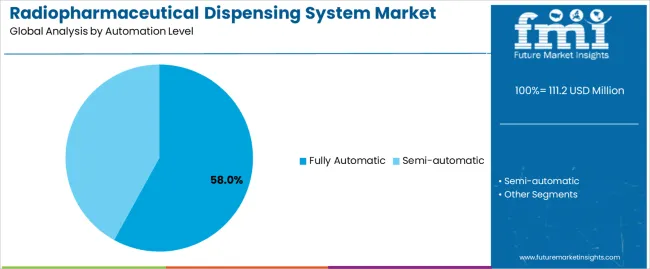
The fully automatic segment represents the dominant force in the radiopharmaceutical dispensing system market, capturing approximately 58% of total market share in 2025. This category delivers comprehensive end-to-end automation with integrated robotics, automated quality control verification, and electronic documentation capabilities. The fully automatic segment's market leadership stems from its ability to eliminate manual handling throughout preparation workflows, superior throughput capacity for high-volume operations, and enhanced traceability supporting pharmaceutical manufacturing compliance and quality assurance requirements.
The semi-automatic segment maintains a substantial 42% market share, serving facilities with moderate procedural volumes that require operator involvement for specific preparation steps while benefiting from automated dose measurement, shielding manipulation, and documentation functions.
Key advantages driving the fully automatic segment include:
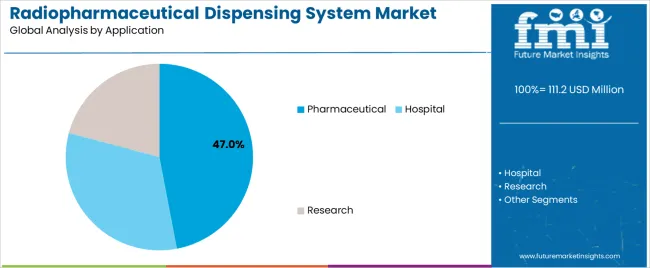
Pharmaceutical applications dominate the radiopharmaceutical dispensing system market with approximately 47% market share in 2025, reflecting the critical need for high-volume production capabilities in centralized commercial radiopharmacies where automated dispensing enables standardized preparation protocols and pharmaceutical manufacturing compliance. The pharmaceutical segment's market leadership is reinforced by widespread adoption across large-scale radiopharmaceutical manufacturers, contract production organizations, and regional distribution centers, which require validated automated systems for quality consistency and regulatory compliance across multi-site operations.
The hospital segment represents 36% market share through in-house nuclear medicine radiopharmacies and on-site preparation facilities that provide patient-specific dose services for diagnostic imaging and therapeutic procedures within integrated healthcare systems. The research segment accounts for 17% market share, featuring specialized dispensing requirements for investigational radiopharmaceutical development, preclinical research programs, and academic nuclear medicine centers.
Key market dynamics supporting application preferences include:
The market is driven by three concrete demand factors tied to operational efficiency and safety enhancement. First, nuclear medicine procedure volume expansion creates increasing requirements for streamlined radiopharmaceutical preparation, with positron emission tomography scans growing 8-12% annually in major healthcare markets according to medical imaging industry data, requiring automated dispensing infrastructure to support rising demand while maintaining preparation quality and delivery timeliness across distributed nuclear medicine facilities. Second, centralized radiopharmacy models drive facilities toward comprehensive automation solutions, with regional production centers serving 10-20 satellite locations becoming standard practice in integrated health systems, creating demand for high-throughput dispensing platforms with sophisticated logistics management and delivery tracking capabilities that ensure dose integrity during transportation. Third, occupational dose reduction mandates incentivize adoption of fully automated equipment, with ALARA principles requiring continuous exposure minimization and modern dispensing systems achieving 70-85% reduction in personnel radiation exposure compared to conventional preparation methods through elimination of direct handling during routine operations.
Market restraints include substantial capital expenditure requirements affecting purchasing timelines and budget allocations, particularly for fully automatic systems where complete installations can exceed USD 500,000 including facility modifications and validation services, posing financial barriers for community hospitals and emerging nuclear medicine programs operating on constrained capital equipment budgets. Implementation complexity regarding facility integration, workflow redesign, and personnel training creates additional challenges, as successful system deployment requires comprehensive process reengineering and staff capability development to transition from manual preparation methods to fully automated workflows while maintaining regulatory compliance and operational continuity during installation phases.
Key trends indicate accelerated adoption in Asia-Pacific markets, particularly China and India, where nuclear medicine infrastructure development and domestic radiopharmaceutical production expansion drive demand for dispensing automation through government healthcare investment programs and pharmaceutical manufacturing localization initiatives. Technology advancement trends toward modular system architectures enabling scalable automation deployment, artificial intelligence-powered quality control verification utilizing advanced radiation detection and image analysis, and cloud-based monitoring platforms enabling remote system supervision and predictive maintenance are enabling next-generation product development. The market could face disruption if alternative radiopharmaceutical delivery models, such as decentralized radioisotope generator systems or point-of-care radiolabeling technologies with comparable quality and regulatory compliance, eliminate the need for centralized high-volume dispensing infrastructure while maintaining equivalent economic efficiency compared to current regional radiopharmacy distribution models.
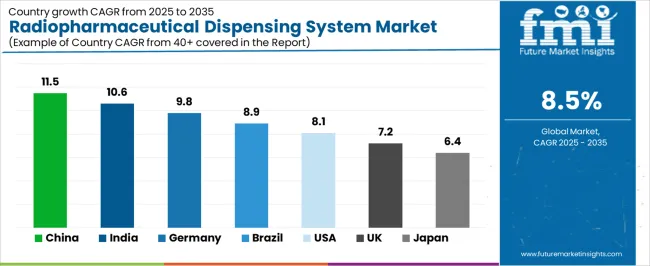
| Country | CAGR (2025-2035) |
|---|---|
| China | 11.5% |
| India | 10.6% |
| Germany | 9.8% |
| Brazil | 8.9% |
| United States | 8.1% |
| United Kingdom | 7.2% |
| Japan | 6.4% |
The radiopharmaceutical dispensing system market is gaining momentum worldwide, with China taking the lead thanks to aggressive nuclear medicine capacity expansion and domestic radiopharmaceutical manufacturing development initiatives. Close behind, India benefits from healthcare infrastructure modernization and nuclear medicine service establishment programs, positioning itself as a strategic growth hub in the Asia-Pacific region. Germany shows strong advancement, where comprehensive radiopharmacy networks and pharmaceutical quality standards strengthen its role in European healthcare systems. Brazil demonstrates robust growth through nuclear medicine modernization and radiopharmaceutical production capacity development, signaling continued advancement in Latin American healthcare infrastructure. Meanwhile, the United States maintains steady progress through theranostic therapy adoption and centralized radiopharmacy network expansion, while the United Kingdom and Japan continue to record consistent advancement driven by radiopharmacy consolidation and automation enhancement programs. Together, China and India anchor the global expansion story, while established markets build stability and technological sophistication into the market's growth path.
The report covers an in-depth analysis of 40+ countries, the top-performing countries are highlighted below.
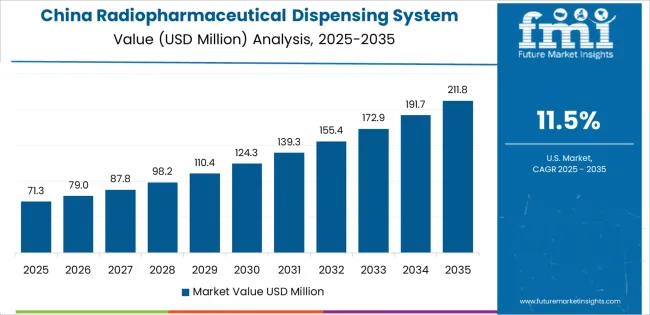
China demonstrates the strongest growth potential in the Radiopharmaceutical Dispensing System Market with a CAGR of 11.5% through 2035. The country's leadership position stems from comprehensive nuclear medicine infrastructure development, intensive radiopharmaceutical manufacturing capacity establishment, and aggressive cancer care network expansion driving adoption of automated dispensing systems. Growth is concentrated in major healthcare and pharmaceutical production centers, including Beijing, Shanghai, Guangzhou, and Chengdu, where hospital nuclear medicine departments, commercial radiopharmacies, and pharmaceutical manufacturers are implementing advanced dispensing technologies for operational efficiency and quality enhancement. Distribution channels through medical equipment distributors, pharmaceutical equipment suppliers, and direct manufacturer relationships expand deployment across tertiary hospitals, regional radiopharmacy centers, and domestic radiopharmaceutical production facilities. The country's Healthy China 2030 strategy provides policy support for nuclear medicine advancement, including investment in radiopharmacy automation and domestic radioisotope production capabilities.
Key market factors:
In major metropolitan healthcare centers, emerging nuclear medicine facilities, and government hospital networks, the adoption of radiopharmaceutical dispensing systems is accelerating across centralized radiopharmacies, hospital nuclear medicine departments, and pharmaceutical production facilities, driven by cancer burden increases and government healthcare infrastructure modernization initiatives. The market demonstrates strong growth momentum with a CAGR of 10.6% through 2035, linked to comprehensive nuclear medicine capacity expansion and increasing focus on operational efficiency enhancement solutions. Indian healthcare and pharmaceutical organizations are implementing automated dispensing equipment and integrated workflow technologies to improve service delivery while meeting international pharmaceutical quality standards for domestic and export markets. The country's National Cancer Control Programme creates sustained demand for nuclear medicine infrastructure, while increasing emphasis on radiation safety drives adoption of automation systems that minimize personnel exposure.
Germany's advanced nuclear medicine sector demonstrates sophisticated implementation of radiopharmaceutical dispensing systems, with documented integration showing 80-90% workflow automation achievement in major centralized radiopharmacies and hospital nuclear medicine departments through comprehensive quality management platforms. The country's healthcare infrastructure in major medical and pharmaceutical centers, including Berlin, Munich, Hamburg, and Frankfurt, showcases integration of fully automatic dispensing technologies with existing radiopharmacy operations, leveraging expertise in pharmaceutical manufacturing and nuclear medicine practice excellence. German healthcare and pharmaceutical organizations emphasize regulatory compliance and process optimization, creating demand for validated automated systems that support European pharmaceutical quality standards and occupational radiation protection requirements. The market maintains strong growth through focus on operational excellence and safety enhancement, with a CAGR of 9.8% through 2035.
Key development areas:
The Brazilian market demonstrates growing implementation of radiopharmaceutical dispensing technologies based on nuclear medicine service expansion and domestic radioisotope production development for enhanced healthcare self-sufficiency. The country shows solid potential with a CAGR of 8.9% through 2035, driven by cancer care infrastructure investment and radiopharmaceutical manufacturing capacity building across major healthcare and pharmaceutical regions, including São Paulo, Rio de Janeiro, Minas Gerais, and Rio Grande do Sul. Brazilian facilities are adopting automated dispensing equipment for compliance with pharmaceutical quality requirements, particularly in government-operated radiopharmacy networks serving public hospitals and in commercial production facilities supplying private healthcare systems. Technology deployment channels through medical equipment distributors, pharmaceutical engineering consultants, and specialized nuclear medicine suppliers expand coverage across diverse healthcare and industrial settings.
Leading market segments:
The USA market demonstrates advanced implementation of radiopharmaceutical dispensing technologies based on centralized radiopharmacy network expansion and theranostic therapy growth for enhanced nuclear medicine capabilities. The country shows solid potential with a CAGR of 8.1% through 2035, driven by alpha-emitter therapy commercialization and integrated health system radiopharmacy consolidation across major healthcare markets, including academic medical centers, cardinal health systems, and commercial radiopharmacy networks. American facilities are adopting comprehensive dispensing automation for compliance with USP pharmaceutical compounding standards, particularly in centralized radiopharmacies requiring Good Manufacturing Practice compliance and in specialty nuclear medicine facilities implementing novel radiopharmaceutical therapy programs. Technology deployment channels through nuclear medicine equipment distributors, radiopharmacy consultants, and direct manufacturer relationships expand coverage across diverse nuclear medicine applications.
Leading market segments:
The United Kingdom's radiopharmaceutical dispensing system market demonstrates mature implementation focused on centralized radiopharmacy models and comprehensive pharmaceutical quality compliance, with documented deployment across 70-75% of NHS radiopharmacy operations through national procurement frameworks. The country maintains steady growth momentum with a CAGR of 7.2% through 2035, driven by NHS nuclear medicine service rationalization and radiopharmacy quality assurance enhancement aligned with Medicines and Healthcare products Regulatory Agency pharmaceutical manufacturing standards. Major healthcare regions, including London, Manchester, Birmingham, and Scotland, showcase consistent deployment of automated dispensing platforms that support MHRA-compliant radiopharmaceutical production requiring comprehensive quality documentation and pharmaceutical manufacturing controls.
Key market characteristics:
Japan's radiopharmaceutical dispensing system market demonstrates sophisticated implementation focused on precision dose preparation and comprehensive quality assurance, with documented integration achieving 85-92% workflow consistency in major university hospital radiopharmacies and commercial production facilities through advanced quality management systems. The country maintains steady growth momentum with a CAGR of 6.4% through 2035, driven by nuclear medicine service excellence and pharmaceutical quality cultural emphasis aligned with strict regulatory compliance standards. Major healthcare and pharmaceutical regions, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase advanced deployment of automated dispensing platforms that integrate with existing radiopharmacy infrastructure and comprehensive pharmaceutical quality control protocols.
Key market characteristics:
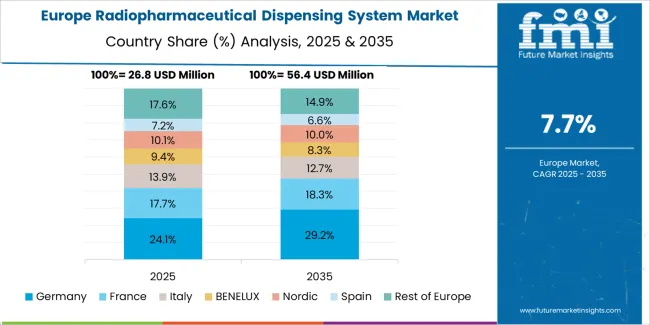
The radiopharmaceutical dispensing system market in Europe is projected to grow from USD 38.8 million in 2025 to USD 80.2 million by 2035, registering a CAGR of 7.5% over the forecast period. Germany is expected to maintain its leadership position with a 30.6% market share in 2025, declining slightly to 29.8% by 2035, supported by its extensive centralized radiopharmacy infrastructure and major pharmaceutical production centers, including Berlin, Munich, and Hamburg operations.
France follows with a 20.4% share in 2025, projected to reach 21.1% by 2035, driven by comprehensive radiopharmacy network consolidation programs and pharmaceutical manufacturing expansion. The United Kingdom holds a 17.8% share in 2025, expected to decrease to 17.2% by 2035 due to mature centralized operations. Italy commands a 12.9% share, while Spain accounts for 9.2% in 2025. The Netherlands maintains 4.6% in 2025, reaching 5% by 2035 on radiopharmaceutical production growth. The Rest of Europe region is anticipated to hold 4.5% in 2025, expanding to 4.8% by 2035, attributed to increasing dispensing system adoption in Nordic countries and emerging Central and Eastern European nuclear medicine programs.
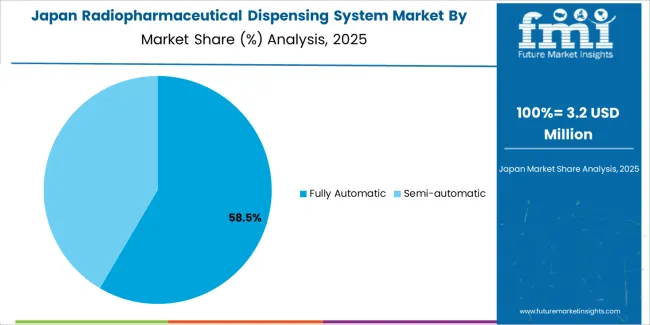
The Japanese radiopharmaceutical dispensing system market demonstrates a mature and quality-focused landscape, characterized by sophisticated integration of fully automatic dispensing platforms and precision quality control systems with existing pharmaceutical production infrastructure across commercial radiopharmacies, university hospital nuclear medicine departments, and radiopharmaceutical manufacturing facilities. Japan's emphasis on pharmaceutical quality excellence and comprehensive documentation drives demand for validated automated systems that support regulatory compliance requirements and quality assurance commitments in radiopharmaceutical preparation. The market benefits from strong partnerships between international system manufacturers and domestic pharmaceutical equipment distributors, creating comprehensive service ecosystems that prioritize equipment reliability and technical support programs. Pharmaceutical and healthcare centers in Tokyo, Osaka, Nagoya, and other major metropolitan areas showcase advanced radiopharmacy implementations where dispensing systems achieve 99% dose accuracy through comprehensive quality control management and precision calibration protocols.
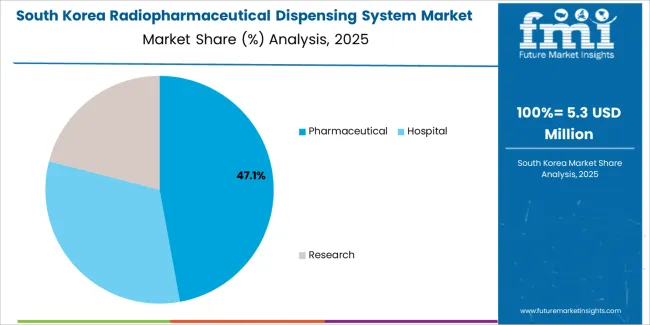
The South Korean radiopharmaceutical dispensing system market is characterized by growing international equipment provider presence, with companies maintaining significant positions through comprehensive installation support and technical services capabilities for hospital and pharmaceutical applications. The market demonstrates increasing emphasis on nuclear medicine quality improvement and radiopharmacy automation enhancement initiatives, as Korean healthcare and pharmaceutical facilities increasingly demand validated automated systems that integrate with domestic pharmaceutical quality management platforms and sophisticated hospital information systems deployed across major medical complexes. Regional pharmaceutical equipment distributors are gaining market share through strategic partnerships with international manufacturers, offering specialized services including Korean regulatory compliance support and comprehensive validation documentation programs for nuclear medicine and pharmaceutical operations. The competitive landscape shows increasing collaboration between multinational dispensing system companies and Korean healthcare technology specialists, creating integrated service models that combine international pharmaceutical quality standards with local nuclear medicine practice requirements and precision healthcare systems.
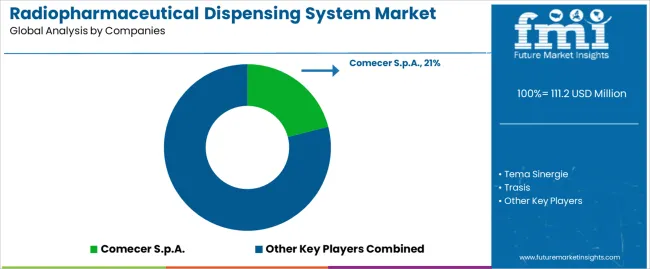
The radiopharmaceutical dispensing system market features approximately 15-20 meaningful players with moderate concentration, where the top three companies control roughly 52-58% of global market share through established radiopharmacy relationships and comprehensive product portfolios. Competition centers on automation sophistication, system reliability, and regulatory compliance support rather than price competition alone. Comecer S.p.A. leads with approximately 21% market share through its comprehensive nuclear medicine equipment and radiopharmacy automation portfolio.
Market leaders include Comecer S.p.A., Tema Sinergie, and Trasis, which maintain competitive advantages through global installation base, extensive validation documentation libraries, and deep expertise in pharmaceutical quality standards and radiation safety regulations across multiple jurisdictions, creating trust and reliability advantages with centralized radiopharmacy operations and pharmaceutical manufacturing facilities. These companies leverage research and development capabilities in automation technology innovation and ongoing technical support relationships to defend market positions while expanding into theranostic applications and specialized radionuclide handling capabilities.
Challengers encompass Norer Shield Medical and Mirion, which compete through specialized dispensing platforms and strong regional presence in key nuclear medicine markets. Technology specialists, including Ultraray Radiation Protection, Von Gahlen, and Lemer Pax, focus on specific automation configurations or application segments, offering differentiated capabilities in modular system designs, customized workflow solutions, and specialized shielding technologies.
Regional players and emerging automation equipment manufacturers create competitive pressure through manufacturing cost advantages and rapid deployment capabilities, particularly in high-growth markets including China and India, where domestic production provides advantages in pricing flexibility and local technical support availability. Market dynamics favor companies that combine comprehensive automation expertise with extensive validation support offerings that address the complete implementation cycle from equipment specification through operational qualification and ongoing compliance maintenance programs.
| Item | Value |
|---|---|
| Quantitative Units | USD 111.2 million |
| Automation Level | Fully Automatic, Semi-automatic |
| Application | Pharmaceutical, Hospital, Research |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | China, India, Germany, Brazil, USA, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | Comecer S.p.A., Tema Sinergie, Trasis, Norer Shield Medical, Mirion, Ultraray Radiation Protection, Von Gahlen, Lemer Pax, Universad Giken, Beijing Zhonghe Yongtai Technology, Bequerel & Sievert, Tianjin Zhongfuan Technology, Beijing Goyuan New Technology, Sunvic |
| Additional Attributes | Dollar sales by automation level and application categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with dispensing system manufacturers and distribution networks, equipment specifications and automation capabilities, integration with radiopharmacy operations and quality management systems, innovations in automation technology and radiation protection platforms, and development of specialized systems with enhanced workflow efficiency and pharmaceutical quality compliance capabilities. |
The global radiopharmaceutical dispensing system market is estimated to be valued at USD 111.2 million in 2025.
The market size for the radiopharmaceutical dispensing system market is projected to reach USD 251.4 million by 2035.
The radiopharmaceutical dispensing system market is expected to grow at a 8.5% CAGR between 2025 and 2035.
The key product types in radiopharmaceutical dispensing system market are fully automatic and semi-automatic.
In terms of application, pharmaceutical segment to command 47.0% share in the radiopharmaceutical dispensing system market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Radiopharmaceutical Market Forecast and Outlook 2025 to 2035
Radiopharmaceutical Logistics Market Analysis Size and Share Forecast Outlook 2025 to 2035
Dispensing Robots Market Size and Share Forecast Outlook 2025 to 2035
Dispensing Guns Market Size and Share Forecast Outlook 2025 to 2035
Dispensing Trays Market Size, Share & Forecast 2025 to 2035
Dispensing Carboy Market Size and Share Forecast Outlook 2025 to 2035
Dispensing Caps Market Growth - Demand & Forecast 2025 to 2035
Key Players & Market Share in the Dispensing Spout Industry
Market Share Breakdown of Dispensing Carboy Providers
Dispensing Spout Market by Cap & Pump Type from 2024 to 2034
Dispensing Jug Market
Dispensing Tap Market
Dispensing Sprayers Market
Dispensing System Market Size and Share Forecast Outlook 2025 to 2035
Beer Dispensing Machine Market Trends - Growth, Demand & Analysis 2025 to 2035
Fluid Dispensing Equipment Market Size and Share Forecast Outlook 2025 to 2035
Twist Dispensing Closures Market Size and Share Forecast Outlook 2025 to 2035
Pouch Dispensing Fitment Market
Hinged Dispensing Caps Market Size and Share Forecast Outlook 2025 to 2035
Competitive Landscape of Hinged Dispensing Caps Providers

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA