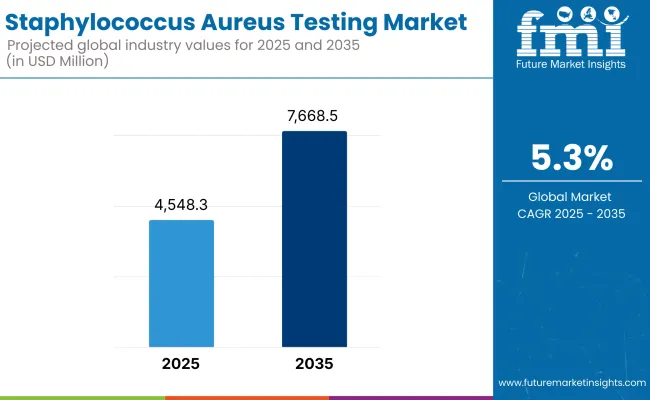
The Staphylococcus aureus testing market is expected to reach approximately USD 4,548.3 million in 2025 and expand to around USD 7,668.5 million by 2035, reflecting a compound annual growth rate (CAGR) of 5.3% over the forecast period.
The staphylococcus aureus testing market is expected to grow steadily between 2025 and 2035, driven by the rising prevalence of healthcare-associated infections (HAIs), community-acquired infections, and increasing demand for rapid diagnostics in clinical microbiology.
Staphylococcus aureus (S. aureus), particularly its methicillin-resistant form (MRSA), is a leading cause of serious infections such as bloodstream infections, pneumonia, skin infections, endocarditis, and post-surgical complications. The increasing global burden of antibiotic resistance has amplified the importance of early detection and strain differentiation, especially in hospital and ICU settings.
The market is supported by advancements in point-of-care (POC) molecular diagnostics, real-time PCR, culture-based techniques, and automated antimicrobial susceptibility testing (AST) platforms. Rapid screening of asymptomatic carriers-especially during hospital admissions and pre-operative care-is becoming standard in many countries. Additionally, growing use of infection surveillance programs, outbreak containment efforts, and precision antimicrobial therapy is driving testing volumes in both public health and private lab sectors.
Key Market Metrics
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 4,548.3 Million |
| Industry Value (2035F) | USD 7,668.5 Million |
| CAGR (2025 to 2035) | 5.3% |
Key market contributors include molecular diagnostics platforms (RT-PCR, LAMP), chromogenic culture media, ELISA kits, and automated blood culture and AST systems. Rapid antigen tests and lateral flow assays are gaining popularity in emergency departments and outpatient settings, while multiplex panels are becoming common in high-throughput labs. Increased hospital surveillance for MRSA colonization, perioperative screening, and routine diagnostic workups for sepsis and wound infections are significantly increasing testing frequency.
North America, with a strong hospital infection surveillance system in the United States and an equally high adoption of molecular diagnostics, is the leader in the global market especially in that MRSA screening has become a pre-surgical requirement across most large health care networks, with national initiatives supervising antimicrobial resistance in the United States supported by public health agencies such as CDC.
Rapid and confirmatory tests are covered by reimbursement in the region, with leading players such as BD, bioMérieux, and Cepheid carrying a considerable share. The high costs associated with various automated testing platforms may restrict access in smaller hospitals and community clinics, thereby providing opportunities for very affordable POC solutions.
Europe has a very attractive market, driven by national infection control regulations and an increasing amount of money being invested in the modernization of hospital microbiology. Countries such as Germany, the UK and the Netherlands lead in the routine screening of S. aureus in both inpatient and outpatient care.
The European Centre for Disease Prevention and Control (ECDC) provides harmonized guidance of antimicrobial resistance testing, contributing to the standardization. The region also has a commendable uptake of chromogenic media, rapid culture diagnostics, and decentralized molecular testing in emergency care settings. However, in some of these markets, differing reimbursement structures and workforce shortages in the public health labs may slow down the adoption of next-gen molecular systems.
Rapidly emerging and growing market, Asia-Pacific has high rates of hospital-acquired infections and growing rates of antibiotic resistance, alongside expanding healthcare infrastructures. Investments in microbiology labs are increasing in China and India, while Japan and South Korea continue to be leading countries in adopting molecular diagnostics.
However, lower- and middle-income countries witness increased demands for the affordable culture and POC screening kits, particularly to diagnose MRSA in intensive care and surgical wards. As a result of various government-led initiatives in controlling AMR, support from WHO and NGOs improve accessibility to diagnostic tools in resource-limited settings. However, there are infrastructural gaps, regulatory fragmentation, and uneven clinical training, which are challenges to full maturity of the market.
Development of rapid, low-cost and multiplex POC testing platforms that detect MRSA and other pathogens at the same time represents an emerging opportunity. AI-powered lab automation systems can increase diagnostic efficiency while also linking them to electronic medical records (EMR) to reduce reporting errors.
The increasing market for wound care in the home, along with community infection testing, provides new pathways to portable diagnostics. Pharmaceutical companies are similarly adding S. aureus screening to clinical trials to monitor infection risk and treatment response. Government and donor-funded AMR surveillance programs are also increasing access to diagnostics in low- and middle-income countries.
There are growing opportunities in the development of rapid, low-cost, and multiplex POC testing platforms capable of detecting MRSA and other pathogens simultaneously. AI-powered lab automation systems, integrated with electronic medical records (EMR), can improve diagnostic efficiency and reduce reporting errors.
The rising demand for home-based wound care and community infection testing opens new channels for portable diagnostics. Pharmaceutical companies are also incorporating S. aureus screening into clinical trials to track infection risk and treatment response. Additionally, government and donor-funded AMR surveillance programs are expanding access to diagnostics in low- and middle-income countries.
Rising Use of Chromogenic Agar for Routine MRSA Screening.
Chromogenic agar is increasingly being used for routine screening of MRSA, becoming popular in healthcare settings as a low-cost and easy-to-use method for screening. The phenotypic basis of direct detection of MRSA organisms has been engineered for use on chromogenic agar media which accounts, in part, for a relative decrease in complexity for identification without complex biochemical tests through the presence of the same-coloured, characteristic representations from colonies of discrete organisms which can be observed in the medium.
It is therefore well suited as a firstline screening tool, particularly in hospital admission contexts where timely identification can mitigate transmission of infection. This method is being used more widely in hospitals and diagnostic laboratories due to its cost-effectiveness, as it requires minimal equipment and gives results in a short time frame.
This innovation in microbiological testing is not only enhancing infection control measures but also alleviating the workload of healthcare facilities, enabling timely microbial identification and improved patient outcomes.
Multiplex Panels for Respiratory and Wound Infections Including MRSA.
Molecular panels of multiplex development for respiratory and wound infection, including MSSA and MRSA, are an emerging trend that could be a remarkable improvement in clinical efficiency. These panels enable the testing of multiple pathogens from a single sample, which is especially relevant for diseases such as pneumonia, wound infections and sepsis, for which MRSA is frequently the causative pathogen.
Incorporating antimicrobial resistance (AMR) markers provides these panels with the additional benefit of also potentially identifying resistance to specific strains, thus informing more focused and appropriate treatment decisions.
This results in a more targeted form of treatment that lessens the need for broad-spectrum antibiotics and their associated risks for antibiotic resistance and side effects. In addition, these panels allow healthcare providers to rapidly identify the causative pathogen of an infection, leading to better patient outcomes due to paved therapy and in a timely manner.
The global Staphylococcus aureus testing market has observed an inclination in growth from 2020 to 2024 due to increasing cognizance about hospital-acquired infection (HAIs), high prevalence rates of MRSA & increasing infection control practices in healthcare settings.
Increasing focus on early-stage infection screening programs along with improvement in diagnostic technologies has led to the high demand for fast and precise testing solutions. The use of molecular diagnostics, principally PCR and newer non-amplified nucleic acid amplification tests (NAATs), in hospital and outpatient settings has cemented this view.
And as MRSA continues to be a serious health care problem, there has been an increased adoption of culture-based and molecular diagnostic tests to provide better management of infections throughout the market. From 2025 to 2035 and beyond, the market is set for acceleration due to improvements in rapid diagnostic platforms, AI-based assays, and the integration of point-of-care testing.
Meanwhile, continued research and progress in AMR and the development of new treatment protocols are expected to influence and complement future testing strategies and simultaneous investments into diagnostic innovations would still pile up the ongoing market expansion.
Comparison Table: 2025 vs. 2035 Projection
| Dimension | 2025 Market State |
|---|---|
| Regulatory Landscape | Strict oversight for MRSA testing in clinical settings, ensuring quality and accuracy in CLIA-certified labs. |
| Technological Advancements | Reliance on culture and PCR-based lab tests, with a turnaround time of 24-48 hours for accurate results. |
| Consumer Demand | Demand focused on hospitals, ICUs, and infection control programs, where timely diagnosis is critical for infection management. |
| Market Growth Drivers | Growth driven by MRSA prevalence, hospital surveillance mandates, and enhanced infection control measures. |
| Sustainability | Testing procedures involve manual reagent handling and moderate levels of biohazardous waste generated by traditional methods. |
| Supply Chain Dynamics | Supply chains remain centralized, dependent on labs for logistics, reagents, and diagnostic equipment. |
| Dimension | 2035 Projection |
|---|---|
| Regulatory Landscape | Broader approval for point-of-care and at-home testing, with regulatory alignment focused on tracking antimicrobial resistance (AMR) at a population level. |
| Technological Advancements | Integration of AI-powered rapid molecular assays, next- gen sequencing (NGS) for resistance typing, and end-to-end sample-to-answer systems for faster and more comprehensive results. |
| Consumer Demand | Increased demand for outpatient, remote, and at-home testing, especially for self-screening in high-risk populations to manage and prevent MRSA outbreaks. |
| Market Growth Drivers | Enhanced by policies for AMR containment, personalized antibiotic stewardship, and the adoption of decentralized diagnostics in outpatient and home settings. |
| Sustainability | Shift to low-waste, cartridge-based diagnostic systems, reusable diagnostic platforms, and environmentally friendly disposables to reduce the environmental impact. |
| Supply Chain Dynamics | More agile and decentralized supply chains, with portable diagnostic hubs, on-demand test distribution, and cloud-based data management for faster results and flexibility. |
Market Outlook
The USA tops the global Staphylococcus aureus testing market on the back of a heavy disease burden from HAIs, especially from MRSA, that has spread throughout institutions. The sustaining of such technology in the area of diagnosis requires a sufficient healthcare infrastructure, of which the country has a fair share.
Awareness about the consequences of putting off treatment and antibiotic resistance has driven investments into rapid testing platforms from both the public and private spheres. Strong government regulations, infection prevention guidelines, and active surveillance programs from agencies like the CDC add to the uptick in market growth.
Increasing numbers of hospitals and diagnostic laboratories are utilizing molecular diagnostic methods (including PCR and nucleic acid amplification tests) for rapid identification and accurate differentiation of S. aureus strains, thus ameliorating patients' outcomes while helping save on healthcare expenditure.
Market Growth Factors
High incidence of MRSA infections in healthcare settings: The increased incidence of MRSA-related hospital infections drives the need for rapid and accurate diagnostic testing to restrict transmission, ensure early treatment, and improve patient safety.
Good presence of leading market players and research institutes: Diagnostic companies and academic institutions, based in the USA, are innovating and collaborating and are speeding up product development and commercialization of advanced Staphylococcus aureus testing solutions.
Governments support multiple initiatives for infection control and prevention: Federal health agencies open the purse to put in place surveillance programs and develop stringent infection control guidelines to usher in the widespread use of diagnostic tools in healthcare establishments.
Advanced diagnostic tools including molecular diagnosis are being adopted: With advances in technology, molecular testing demonstrates superior sensitivity and reduced turnaround time, allowing for timely therapeutic intervention and aiding in formulating effective infection management strategies in clinical settings.
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
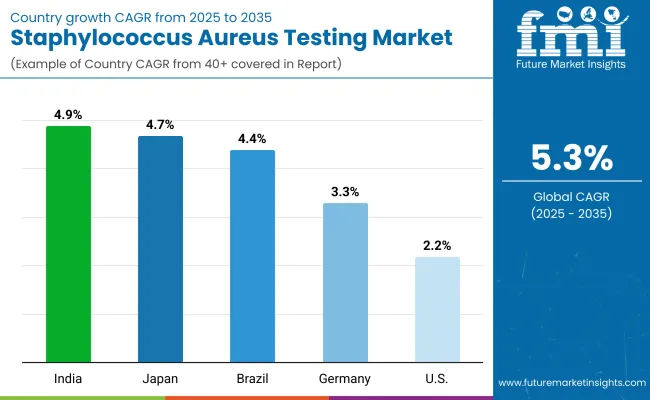
| 2025 to 2035 | 2.2% |
Market Outlook
Germany is a prime country in Europe for Staphylococcus aureus testing, characterized by advanced and highly regulated healthcare systems. Their practices for infection control, in concert with the Robert Koch Institute, ensure systematic screening and diagnosis of hospital-acquired infections related to Staphylococcus aureus. Public health initiatives against antimicrobial resistance have provided impetus for the acceptance of the molecular and rapid diagnostic frontiers. Detection and confirmation in the German healthcare system favor early interventions, including in wards at risk, such as ICUs and surgical wards. Collaborations between diagnostic companies and healthcare institutions are also stimulating innovations in the area, fast tracking the introduction of new testing solutions into clinical practice.
Market Growth Factors
Growing prevalence of antibiotic-resistant infections: Increasing threats by MRSA will certainly witness increased demands for accurate diagnostics to ensure rapid identification and antimicrobial treatment of such patients at least from the time of admission.
Government policies favoring routine screening and surveillance: Public health policies allowing for routine screening in hospitals will augment early detection measures and subsequently support appropriate infection control strategies and limit bacterial transmission.
High acceptance of rapid tests: Rapid tests including PCR and lateral flow assay are being increasingly adopted in hospitals and laboratories in Germany to enhance promptness and therefore the working of clinical decision-making and patient outcome.
Partnerships between health service providers and diagnostic companies: Strategic alliances back the co-development and validation of innovative diagnostics designed to meet the local clinical needs and regulatory landscape.
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| 2025 to 2035 | 3.3% |
Market Outlook.
Steady Growth in Japan's Staphylococcus Aureus Testing Market - Demographically driven, with effectiveness in Healthcare Delivery and active measures in infection control. One of the rapidly aging populations, increasing risk to hospital-acquired infection (HAI), calls for more diagnostic solutions that are timely and accurate.
The universal healthcare system of the country has quality care with a strong emphasis on early detection. Molecular and rapid testing technologies will probably be integrated into the clinical environment. Continual upgrades in hospital diagnostics are also being encouraged through public health policy while private and government sectors invest significantly in infection surveillance and control programs.
With Japan most likely taking an advanced approach to high-precision, minimally invasive diagnostic tools, it will foster development in the market for even better identification and management of S. aureus-related conditions.
Market Growth Factors
Increase awareness of HAIs and their consequences for the public health environment: Awareness and Concern for hospital-acquired infections, such that they are already growing. This creates a high demand for early, accurate testing for S. aureus to avert complications and increase patient safety.
Initiatives of the government for early detection and handling of infections: health authorities in Japan motivates proactive diagnostic screening programs in hospitals to support an early stage detection and prevent escalation of antibiotic resistance.
Advanced diagnostic apparatus within the healthcare institutions:Currently, hospitals and clinics are adopting advanced molecular diagnostics apart from point-of-care tests, thereby shortening the time and improving the precision of diagnosis concerning Staphylococcus aureus.
Doctorate collaboration with the diagnostic companies: The research partnership provides an avenue for establishing innovation in the diagnostic technologies to enable the development and clinical validation of next generation tests specific to the healthcare needs in Japan.
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| 2025 to 2035 | 4.7% |
Market Outlook
India's Staphylococcus aureus testing industry is at high growth due to rising hospital-acquired infections (HAIs), increasing investments in healthcare, and the changing healthcare infrastructure of urban and semi-urban areas.
Antimicrobial resistance, particularly in the methicillin-resistant strains of Staphylococcus aureus (MRSA), is a problem of great public health concern; thus, there is a pressing need for real-time and point-of-care detection of such infections. Initiatives of the government such as Ayushman Bharat and specific public-private partnerships will widen access to quality health care and diagnostics.
At the same time, these developments increase the number of private laboratories and multispecialty hospitals, which maneuver the use and adoption of advanced methods, especially molecular techniques. With compulsory cost-effective measures on the rise concerning infection control, the future of diagnostics in India is about to undergo a disruptive change, opening up multiple avenues for both domestic and global players in the S. aureus testing field.
Market Growth Factors
Heavy burden from infectious diseases, one of which is MRSA: India has a dire problem of antibiotic-resistant infections, which drives increased demand for Staphylococcus aureus testing for proper and early treatment and containment.
Government initiatives to improve the access and quality of healthcare: For instance, Ayushman Bharat and state health missions are some of the programs that have public-wide diagnostic services and early detection capabilities at public health facilities and their infection management potential.
Increasing number of private health facilities with advanced diagnostics: The rapid expansion of private hospitals and labs increased the availability of advanced diagnostic technologies for the quick identification of S. aureus infections.
Increasing demand for low-cost and rapid diagnostic tests: An increased need for cost-effective, point-of-care, and rapid testing solutions is growing, especially in tier 2 and tier 3 cities where diagnostic access remains limited.
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| 2025 to 2035 | 4.9% |
Market Outlook
The Brazil Staphylococcus aureus testing market is booming owing to the bigger and a diverse patient population, increasing healthcare spending and greater focus on fighting against HAIs in the country. With a focus on the public healthcare system (SUS) and a rapidly growing private healthcare sector, advanced diagnostic tools are being increasingly leveraged to enhance early detection and treatment outcomes.
Antimicrobial resistance including that of methicillin-resistant Staphylococcus aureus (MRSA) is an increasing dilemma, such that diagnostic testing is now strategically important in urban hospitals and rural healthcare institutions.
(This is also why we are expanding laboratory infrastructure and training programs that will boost diagnostics across the country.) The evolution of the market towards high-sensitivity, rapid, and cost-effective diagnostic solutions is further buoyed by supportive government policies and growing public awareness regarding infection control.
Market Growth Factors
Why the write-up says that S. aureus and associated infection rates have been decreasing: Due to the increasing occurrence of drug-resistant strains of S. aureus in hospitals, there is increasing demand for reliable testing to permit early detection so that control measures may be instituted.
Government programs targeting improvement in health care delivery: Public health programs directed at modernization of infrastructure and access to diagnostics support early detection and prevention of S. aureus infections.
Increased awareness among health care practitioners regarding infection control: Training and education are enabling clinicians to give priority to early testing and appropriate infection control measures so as to reduce health care-associated infection rates.
The growth of the private sector in the area of advanced diagnostics: The rise of private clinics and diagnostic chains equipped with advanced technology is facilitating great accessibility to quality testing, primarily into urban and semi-urban areas.
Market Forecast
| Year | CAGR (2025 to 2035) |
|---|---|
| 2025 to 2035 | 4.4% |
Nucleic Acid Amplification Tests (NAATs) Dominated The Market Due To Their High Sensitivity.
Because it has the highest diagnostic accuracy and rapidness, the Nucleic Acid Amplification Tests (NAATs) for Staphylococcus aureus testing activity holds the highest market share. PCR and like tests are capable of detecting only minute amounts of bacterial DNA, thus enabling very rapid and valid identifications of methicillin-sensitive and methicillin-resistant strains (MSSA and MRSA, respectively).
This capability is particularly necessary at the clinical level where late or incorrect diagnosis can have deleterious consequences. Additionally, one of the main uses of NAATs would be to facilitate the deployment of antimicrobial targeted therapy through the differentiation of resistant strains, aiding effective infection control. The increasing demand for real-time, high-throughput results in hospitals, diagnostic laboratories, as well as technological advancements, and reduced cost of tests continue to fuel the adoption of NAATs.
Agar-Based Tests Dominated The Market Due To Their Cost-Effectiveness And Widespread Availability
Agar medium-based tests lead the Staphylococcus aureus testing market as they provide a cost-effective and robust method for bacterial culture and for antibiotic susceptibility testing. Common tests employed in the initial screening and identification of S. aureus comprise the mannitol salt agar or chromogenic agar, which are routinely performed in public and private laboratories.
They have no requirement of sophisticated equipment, can be high throughput and are effective in isolating and differentiating resistant strains like MRSA [10-12]. Given these reasons, we consider them an ideal candidate for high throughput environments like developing countries and public health facilities.
Agar-based testing is certainly slower than molecular methods but is the gold standard of the microbiology lab due to its low cost and provision of detailed phenotypic information, to support both clinical decision-making and infection control programmes.
The clinical segment dominated the market due to the high demand for accurate and timely diagnosis of Staphylococcus aureus infections in hospitals and healthcare facilities.
The clinical application segment held the largest share of the Staphylococcus aureus testing market, attributed to the growing demand for timely and accurate diagnostic solutions in hospitals, clinics, and diagnostic laboratories. Staphylococcus aureus, especially methicillin-resistant strains (MRSA), is a major threat in clinical settings as patients are more likely to be prone to hospital-acquired infections (HAIs).
Diagnosis of such infections may be significant to ensure proper use of antibiotics, course prevention of prancing, and decrease in morbidity and mortality of afflicted patients. Thus, this method of fast diagnosis and containment using culture-based and molecular approaches is now preferred by health care providers.
The higher acceptance of this monitoring within ICUs, operating theaters and long-term care homes will additionally work in the favor of increasing the demand, in a way strengthening the position of clinical segment inoveralltesting matrix.
The pharmaceutical segment dominated the market due to growing demand for Staphylococcus aureus testing in drug development, vaccine research, and antibiotic efficacy studies.
Increasing demand for the accurate identification of microbes in applications such as drug discovery, vaccine development, and determination of antimicrobial resistance mechanisms has further accelerated the pharmaceutical application segment in the overall Staphylococcus aureus testing market.
Pharmaceutical companies rely on accurate tests to measure the effectiveness of new antibiotics and formulations against both methicillin-sensitive and resistant strains of S. aureus. Despite the global threat AMR poses, pharmaceutical R&D has attention-directed now to creating therapeutics that will act narrowly and preventative modalities like vaccines.
Staphylococcus aureus detection methods are of extremely significant importance in preclinical and clinical trial phases for bacterial load, drug sensitivity and treatment response. Sustained and continued reliance on research in this segment keeps it as the fastest growingand leading in the market.
The Staphylococcus aureus testing market competitive analysis can be characterized by vigorous innovation, partnerships, and growing emphasis on rapid and high-accuracy solutions. With rising demand for early and accurate detection of MRSA and MSSA infections, players in the market are developing advanced molecular diagnostics and point-of-care testing kits.
With affordable and scalable screening solutions, companies are growing their global footprints in developing countries. Continuous product innovation and validation is made possible through collaborations with research institutions and healthcare providers. Additionally, regulatory approvals and quality accreditations are especially important differentiators in this highly regulated market.
Efforts to bring automated platforms into the clinic and to offer comprehensive panels of tests, which can improve speed and throughput of diagnostics, are also shaping the competition. Pricing strategy, technology evolution, and portfolio diversification continued to serve as key competitive levers across the regions.
Market Share Analysis by Company
| Company Name | Estimated Market Share (%) |
|---|---|
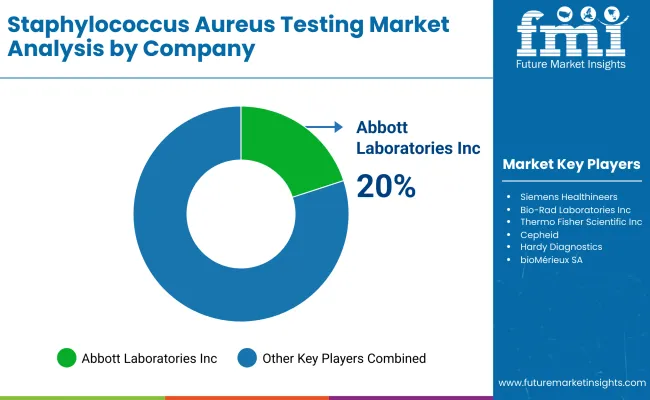
| Abbott Laboratories Inc. | 20-25% |
| Siemens Healthineers | 15-20% |
| Bio-Rad Laboratories Inc. | 10-15% |
| Thermo Fisher Scientific Inc. | 8-12% |
| Roche Diagnostics | 5-10% |
| Others | 15-20% |
| Company Name | Key Offerings/Activities |
|---|---|
| Abbott Laboratories Inc. | Offers a range of diagnostic solutions, including rapid tests and molecular assays for the detection of Staphylococcus aureus and MRSA strains. |
| Siemens Healthineers | Provides advanced laboratory diagnostic equipment and assays for the identification of bacterial pathogens, including Staphylococcus aureus. |
| Bio-Rad Laboratories Inc. | Develops and supplies a variety of diagnostic products, including culture media and molecular testing kits for Staphylococcus aureus detection. |
| Thermo Fisher Scientific Inc. | Offers comprehensive diagnostic tools, including PCR-based assays and culture media, for accurate identification of Staphylococcus aureus. |
| Roche Diagnostics | Provides molecular diagnostic solutions, including real-time PCR assays, for the rapid and precise detection of Staphylococcus aureus infections. |
| Others | Companies like Cepheid and Hardy Diagnostics contribute to the market with specialized diagnostic platforms and culture media products. |
Key Company Insights
Abbott Laboratories Inc.
A global leader in diagnostics, Abbott offers a comprehensive portfolio of testing solutions, including rapid lateral flow assays and molecular diagnostics, to detect Staphylococcus aureus and its resistant strains.
Siemens Healthineers
Known for its innovative diagnostic technologies, Siemens provides automated laboratory systems and assays that enhance the efficiency and accuracy of bacterial pathogen detection.
Bio-Rad Laboratories Inc.
With a strong focus on research and development, Bio-Rad offers a wide array of diagnostic products, including culture media and molecular testing kits, catering to clinical laboratories worldwide.
Thermo Fisher Scientific Inc.
Thermo Fisher's extensive product range includes advanced PCR-based assays and culture media, supporting clinical laboratories in the rapid identification of Staphylococcus aureus.
Roche Diagnostics: Roche's molecular diagnostic solutions, such as real-time PCR assays, provide healthcare professionals with reliable tools for the early detection and management of Staphylococcus aureus infections.
These companies contribute to the Staphylococcus aureus testing market through innovative diagnostic solutions, enhancing the ability of healthcare providers to detect and manage infections effectively.
Coagulase test, ancillary tests, agar-based tests and nucleic acid amplification tests.
Pharmaceutical, clinical, food testing and other.
Hospitals, diagnostic clinics, food testing laboratories and others.
North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa.
The global Staphylococcus aureus testing industry is projected to witness CAGR of 5.3% between 2025 and 2035.
The global Staphylococcus aureus testing industry stood at USD 4,319.4 million in 2024.
The global Staphylococcus aureus testing industry is anticipated to reach USD 7,668.5 million by 2035 end.
China is expected to show a CAGR of 5.3% in the assessment period.
The key players operating in the global Staphylococcus aureus testing industry are Abbott Laboratories Inc., Siemens Healthineers, Bio-Rad Laboratories Inc., Thermo Fisher Scientific Inc., Roche Diagnostics, Cepheid, Hardy Diagnostics, bioMérieux SA, Becton, Dickinson and Company (BD) and Others.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 5: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 9: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 10: Latin America Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 13: Western Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Western Europe Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 15: Western Europe Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 16: Western Europe Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 17: Eastern Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 18: Eastern Europe Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 19: Eastern Europe Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 20: Eastern Europe Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 21: South Asia and Pacific Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: South Asia and Pacific Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 23: South Asia and Pacific Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 24: South Asia and Pacific Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 25: East Asia Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 26: East Asia Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 27: East Asia Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 28: East Asia Market Value (US$ Million) Forecast by End User, 2018 to 2033
Table 29: Middle East and Africa Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 30: Middle East and Africa Market Value (US$ Million) Forecast by Test Type, 2018 to 2033
Table 31: Middle East and Africa Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 32: Middle East and Africa Market Value (US$ Million) Forecast by End User, 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Application, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by End User, 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 5: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 9: Global Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 10: Global Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 11: Global Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 12: Global Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 13: Global Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 14: Global Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 15: Global Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 16: Global Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 17: Global Market Attractiveness by Test Type, 2023 to 2033
Figure 18: Global Market Attractiveness by Application, 2023 to 2033
Figure 19: Global Market Attractiveness by End User, 2023 to 2033
Figure 20: Global Market Attractiveness by Region, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 22: North America Market Value (US$ Million) by Application, 2023 to 2033
Figure 23: North America Market Value (US$ Million) by End User, 2023 to 2033
Figure 24: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 25: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 26: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 27: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 28: North America Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 29: North America Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 30: North America Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 37: North America Market Attractiveness by Test Type, 2023 to 2033
Figure 38: North America Market Attractiveness by Application, 2023 to 2033
Figure 39: North America Market Attractiveness by End User, 2023 to 2033
Figure 40: North America Market Attractiveness by Country, 2023 to 2033
Figure 41: Latin America Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 42: Latin America Market Value (US$ Million) by Application, 2023 to 2033
Figure 43: Latin America Market Value (US$ Million) by End User, 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 45: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 49: Latin America Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 50: Latin America Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 52: Latin America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 53: Latin America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 55: Latin America Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 56: Latin America Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 57: Latin America Market Attractiveness by Test Type, 2023 to 2033
Figure 58: Latin America Market Attractiveness by Application, 2023 to 2033
Figure 59: Latin America Market Attractiveness by End User, 2023 to 2033
Figure 60: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 61: Western Europe Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 62: Western Europe Market Value (US$ Million) by Application, 2023 to 2033
Figure 63: Western Europe Market Value (US$ Million) by End User, 2023 to 2033
Figure 64: Western Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 65: Western Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 66: Western Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 67: Western Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 68: Western Europe Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 69: Western Europe Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 70: Western Europe Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 71: Western Europe Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 72: Western Europe Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 73: Western Europe Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 74: Western Europe Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 75: Western Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 76: Western Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 77: Western Europe Market Attractiveness by Test Type, 2023 to 2033
Figure 78: Western Europe Market Attractiveness by Application, 2023 to 2033
Figure 79: Western Europe Market Attractiveness by End User, 2023 to 2033
Figure 80: Western Europe Market Attractiveness by Country, 2023 to 2033
Figure 81: Eastern Europe Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 82: Eastern Europe Market Value (US$ Million) by Application, 2023 to 2033
Figure 83: Eastern Europe Market Value (US$ Million) by End User, 2023 to 2033
Figure 84: Eastern Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 85: Eastern Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 86: Eastern Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 87: Eastern Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 88: Eastern Europe Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 89: Eastern Europe Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 90: Eastern Europe Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 91: Eastern Europe Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 92: Eastern Europe Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 93: Eastern Europe Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 94: Eastern Europe Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 95: Eastern Europe Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 96: Eastern Europe Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 97: Eastern Europe Market Attractiveness by Test Type, 2023 to 2033
Figure 98: Eastern Europe Market Attractiveness by Application, 2023 to 2033
Figure 99: Eastern Europe Market Attractiveness by End User, 2023 to 2033
Figure 100: Eastern Europe Market Attractiveness by Country, 2023 to 2033
Figure 101: South Asia and Pacific Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 102: South Asia and Pacific Market Value (US$ Million) by Application, 2023 to 2033
Figure 103: South Asia and Pacific Market Value (US$ Million) by End User, 2023 to 2033
Figure 104: South Asia and Pacific Market Value (US$ Million) by Country, 2023 to 2033
Figure 105: South Asia and Pacific Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 106: South Asia and Pacific Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 107: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 108: South Asia and Pacific Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 109: South Asia and Pacific Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 110: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 111: South Asia and Pacific Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 112: South Asia and Pacific Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 113: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 114: South Asia and Pacific Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 115: South Asia and Pacific Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 116: South Asia and Pacific Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 117: South Asia and Pacific Market Attractiveness by Test Type, 2023 to 2033
Figure 118: South Asia and Pacific Market Attractiveness by Application, 2023 to 2033
Figure 119: South Asia and Pacific Market Attractiveness by End User, 2023 to 2033
Figure 120: South Asia and Pacific Market Attractiveness by Country, 2023 to 2033
Figure 121: East Asia Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 122: East Asia Market Value (US$ Million) by Application, 2023 to 2033
Figure 123: East Asia Market Value (US$ Million) by End User, 2023 to 2033
Figure 124: East Asia Market Value (US$ Million) by Country, 2023 to 2033
Figure 125: East Asia Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 126: East Asia Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 127: East Asia Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 128: East Asia Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 129: East Asia Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 130: East Asia Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 131: East Asia Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 132: East Asia Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 133: East Asia Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 134: East Asia Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 135: East Asia Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 136: East Asia Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 137: East Asia Market Attractiveness by Test Type, 2023 to 2033
Figure 138: East Asia Market Attractiveness by Application, 2023 to 2033
Figure 139: East Asia Market Attractiveness by End User, 2023 to 2033
Figure 140: East Asia Market Attractiveness by Country, 2023 to 2033
Figure 141: Middle East and Africa Market Value (US$ Million) by Test Type, 2023 to 2033
Figure 142: Middle East and Africa Market Value (US$ Million) by Application, 2023 to 2033
Figure 143: Middle East and Africa Market Value (US$ Million) by End User, 2023 to 2033
Figure 144: Middle East and Africa Market Value (US$ Million) by Country, 2023 to 2033
Figure 145: Middle East and Africa Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 146: Middle East and Africa Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 147: Middle East and Africa Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 148: Middle East and Africa Market Value (US$ Million) Analysis by Test Type, 2018 to 2033
Figure 149: Middle East and Africa Market Value Share (%) and BPS Analysis by Test Type, 2023 to 2033
Figure 150: Middle East and Africa Market Y-o-Y Growth (%) Projections by Test Type, 2023 to 2033
Figure 151: Middle East and Africa Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 152: Middle East and Africa Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 153: Middle East and Africa Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 154: Middle East and Africa Market Value (US$ Million) Analysis by End User, 2018 to 2033
Figure 155: Middle East and Africa Market Value Share (%) and BPS Analysis by End User, 2023 to 2033
Figure 156: Middle East and Africa Market Y-o-Y Growth (%) Projections by End User, 2023 to 2033
Figure 157: Middle East and Africa Market Attractiveness by Test Type, 2023 to 2033
Figure 158: Middle East and Africa Market Attractiveness by Application, 2023 to 2033
Figure 159: Middle East and Africa Market Attractiveness by End User, 2023 to 2033
Figure 160: Middle East and Africa Market Attractiveness by Country, 2023 to 2033






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
MRSA Testing Market Insights – Growth & Forecast 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
Eye Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HSV Testing Market Size and Share Forecast Outlook 2025 to 2035
IoT Testing Equipment Market Size and Share Forecast Outlook 2025 to 2035
HPV Testing and Pap Test Market Size and Share Forecast Outlook 2025 to 2035
GMO Testing Services Market Insights – Food Safety & Regulatory Compliance 2024 to 2034
GMP Testing Services Market
LTE Testing Equipment Market Growth – Trends & Forecast 2019-2027
Drug Testing Systems Market Size and Share Forecast Outlook 2025 to 2035
Sand Testing Equipments Market Size and Share Forecast Outlook 2025 to 2035
Tire Testing Machine Market Size and Share Forecast Outlook 2025 to 2035
Self-Testing Market Analysis - Size, Share, and Forecast 2025 to 2035
Food Testing Services Market Size, Growth, and Forecast for 2025–2035
Bend Testing Machine Market Growth - Trends & Forecast 2025 to 2035
An Analysis of the Leak testing Machine Market by Detectors and Sensors Hardware Type through 2035
Soil Testing Market Growth - Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA