The Anesthesia Ultrasound Systems Market is valued at USD 348.8 million in 2025. As per FMI's analysis, the Anesthesia Ultrasound Systems Industry will grow at a CAGR of 7.4% and reach USD 723.3 million by 2035.
In 2024, growth in anesthesia ultrasound systems was driven by an uptick in regional anesthesia procedures, particularly in orthopedic and cardiovascular surgeries. Hospitals across North America and Western Europe prioritized the acquisition of high-resolution ultrasound systems to improve nerve block accuracy and reduce complications, aligning with evolving clinical guidelines. Demand also surged in ambulatory surgical centers, where portable and compact systems gained traction for their ease of use and operational efficiency.
FMI analysis found that AI-powered enhancements, including real-time image optimization and needle tracking, improved procedural confidence and expanded the user base among general practitioners and novice anesthesiologists. In parallel, healthcare investments in Asia-Pacific, especially in secondary care facilities across India and China, led to the rapid deployment of cost-sensitive models to broaden access to image-guided anesthesia.
Looking ahead, 2025 is expected to usher in wider adoption across emerging economies, supported by training programs and public-private procurement schemes. FMI opines that manufacturers focusing on automation, compact system design, and clinician-friendly interfaces will dominate commercial opportunities as the segment shifts toward precision-based anesthesia delivery.
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 348.8 million |
| Industry Value (2035F) | USD 723.3 million |
| CAGR (2025 to 2035) | 7.4% |
The Anesthesia Ultrasound Systems Industry is on a steady growth trajectory, driven by the rising preference for precision-guided regional anesthesia and minimally invasive procedures. FMI analysis found that increasing surgical volumes, especially in orthopedics and cardiovascular care, are pushing healthcare providers to adopt ultrasound systems that enhance safety and efficiency. Manufacturers offering AI-enabled, portable solutions stand to benefit the most, while vendors slow to innovate risk losing relevance in this rapidly evolving clinical space.
Prioritize Product Affordability and Portability
Executives should invest in developing cost-effective, compact ultrasound systems tailored for secondary care facilities and emerging industries to drive wider adoption and expand industry share.
Align with AI and Workflow Integration Trends
Focus R&D efforts on integrating AI-powered features such as automated nerve detection and real-time image enhancement to meet clinician demand for precision, speed, and ease of use.
Expand Strategic Partnerships and Training Networks
Strengthen distribution through local partnerships and invest in clinical training programs to increase user proficiency, especially in high-growth regions across Asia-Pacific and Latin America.
| Risk | Probability - Impact |
|---|---|
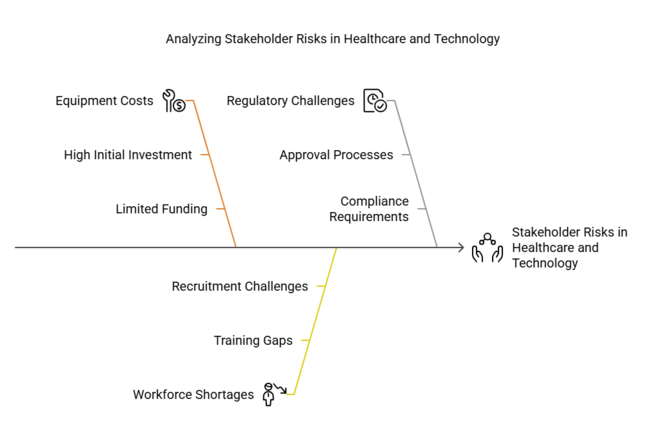
| High equipment costs limiting adoption in low-resource settings | Medium - High |
| Shortage of trained anesthesiologists skilled in ultrasound-guided procedures | High - Medium |
| Regulatory delays for AI-integrated systems in key industri es | Medium - Medium |
| Priority | Immediate Action |
|---|---|
| Explore strategic partnerships | Accelerate AI integration in ultrasound systems through collaborations |
| Conduct industry analysis | Identify untapped opportunities in emerging economies for anesthesia ultrasound systems |
| Develop a robust training program | Support widespread adoption of ultrasound-guided regional anesthesia |
| Invest in compact and portable models | Cater to ambulatory surgical centres with affordable and portable ultrasound systems |
| Enhance product affordability | Implement cost-effective manufacturing strategies for emerging industries |
To stay ahead, companies must prioritize the development of AI-powered ultrasound systems that offer enhanced precision and real-time image optimization. The focus should be on creating compact, portable models tailored for emerging industries and ambulatory surgical centres, where demand is rapidly increasing due to their affordability and operational efficiency.
Strategic partnerships, particularly in Asia-Pacific, should be strengthened to expand distribution channels and accelerate training programs. By aligning product offerings with evolving clinical needs and leveraging AI for workflow integration, companies can differentiate themselves, driving industry share growth and leading the industry in innovation and accessibility. This approach will set the stage for sustained growth through 2035.
| Countries | Policies, Regulations, and Certifications |
|---|---|
| USA | The FDA regulates anesthesia ultrasound systems, requiring FDA 510(k) clearance for most devices or Pre market Approval (PMA) for higher-risk devices. Compliance with HIPAA is necessary for patient data privacy. |
| UK | Regulated by the MHRA. Anesthesia ultrasound systems must meet the UKCA mark certification post-Brexit. Compliance with the CE mark i s necessary in Northern Ireland. |
| France | The ANSM governs medical devices and enforces compliance with the CE certification as per EU MDR. Data privacy is managed under GDPR regulations. |
| Germany | The BfArM requires devices to comply with EU MDR and obtain CE marking. GDPR compliance is mandatory for the protection of patient data. |
| Italy | The Ministry of Health ensures that anesthesia ultrasound systems are CE-marked under EU MDR. Compliance with GDPR is also required. |
| South Korea | The KFDA regulates the approval of medical devices, and anesthesia ultrasound systems must meet the K-TR certification for safety an d electromagnetic compatibility. |
| Japan | The PMDA oversees medical device approval, requiring compliance with Japanese Industrial Standards (JIS). The J-MHLW (Ministry of Health, Labor and Welfare) is also involve d in the certification process. |
| China | The NMPA (formerly CFDA) regulates the approval and industry entry of medical devices. China Compulsory Certification (CCC) is necessary for product safety. Compliance with China's Cybersecurity La w is essential for patient data. |
| Australia-NZ | In Australia, the TGA and in New Zealand, MedSafe regulate anesthesia ultrasound systems, requiring compliance with TGA approval and MedSafe registration. Both countries follow CE certificati on and data privacy regulations. |
The transversus abdominis plane (TAP) block is anticipated to grow at the highest CAGR of 8.1% between 2025 and 2035. This segment is gaining traction due to its effectiveness in providing analgesia for abdominal surgeries, including C-sections, hernia repairs, and laparoscopic procedures. TAP blocks help reduce the use of opioids post-surgery, aligning with global trends toward opioid-sparing anesthesia.
Technological improvements in ultrasound imaging have further facilitated the safe and accurate administration of this block. As more surgical procedures are performed on an outpatient basis, particularly abdominal surgeries, the TAP block is increasingly being adopted due to its reliability, safety, and patient comfort. These factors make it the fastest-growing application segment in the industry.
Linear probes are projected to maintain their dominance as the leading product segment with a CAGR of 7.9% from 2025 to 2035. This is owing to their high-resolution imaging, which makes them extremely useful for superficial nerve blocks commonly used in upper limb and vascular access procedures. Within both hospital and ambulatory settings their precision and ease of handling experienced them to be the preferred choice.
The increasing use of ultrasound-guided regional anesthesia (UGRA), particularly in orthopedic and outpatient surgeries, justifies the sustained adoption of linear probes. In addition, repeat purchases and upgrades are driven by consistent improvements in probe ergonomics and imaging quality. Strong evidence-based development and implementation of linear probes will be central to both relational growth in UGRA as well as procedural results in training as UGRA emerges as a global phenomenon.
Ambulatory surgical centers (ASCs) are the fastest-growing end-user segment, with a projected CAGR of 8.3% from 2025 to 2035. The increasing shift toward outpatient surgical procedures-due to shorter recovery times, reduced healthcare costs, and growing patient preference-is a key growth driver. ASCs are highly receptive to technology that enables quicker turnaround and better pain management, making them ideal adopters of ultrasound-guided anesthesia systems.
These centres benefit from the compact, portable nature of modern ultrasound devices, allowing anesthesiologists to perform nerve blocks efficiently. With global healthcare systems encouraging cost-effective surgical models, ASCs will continue to rise, significantly contributing to the expansion of the anesthesia ultrasound industry.
Sales inUnited States is anticipated to grow at a CAGR of 8.2% between 2025 and 2035, outperforming the global average due to favourable reimbursement structures and increased outpatient surgical procedures. The country has witnessed strong momentum in the adoption of ultrasound-guided regional anesthesia (UGRA), driven by the expansion of ambulatory surgical centres (ASCs) and chronic pain management clinics.
The presence of established medical device players, a mature healthcare infrastructure, and growing awareness about precision-guided anesthesia delivery contribute to the USA leading the global industry. Additionally, regulatory clarity from the Centres for Medicare & Medicaid Services (CMS) supports consistent demand and innovation.
The industry in United Kingdom is expected to grow at a CAGR of 6.8% from 2025 to 2035, slightly below the global average, due to fiscal pressures in the public healthcare sector. The National Health Service (NHS) supports cost-effective implementation of UGRA, especially in orthopedic and day-case surgeries, which enhances patient recovery and reduces hospital stays.
However, budgetary constraints and limited training in advanced anesthesia techniques slow industry penetration. Despite these barriers, rising interest in low-cost, portable ultrasound units for anesthesia in rural and secondary care settings could foster moderate growth, especially as private sector involvement in surgical services increases.
Anesthesiaultra sound systems industry in France is anticipated to grow at a CAGR of 6.5% over 2025 to 2035, showing steady but restrained industry expansion. The country benefits from a well-established public hospital network, where anesthesia ultrasound systems are being increasingly adopted for regional blocks and labour analgesia.
However, sluggish investment in private hospitals and a relatively slower pace of technological change in surgical workflows limit accelerated growth. The reimbursement environment is evolving, and France’s adherence to EU safety standards enhances equipment standardization. Growth may further be supported by clinical evidence supporting the efficacy of UGRA, especially among aging populations requiring orthopedic and oncological surgeries.
Germany sales are expected to grow at a CAGR of 7.1% from 2025 to 2035, slightly below the USA but above most European peers. It remains the largest and most advanced EU industry for anesthesia ultrasound systems, with a strong emphasis on precision surgery and high rates of UGRA training among anesthetists.
German hospitals are early adopters of imaging-integrated surgical workflows, creating fertile ground for anesthesia ultrasound expansion. Additionally, stringent quality regulations encourage standardized ultrasound usage. Academic research and surgeon preference for image-guided blocks further enhance system sales. However, pricing and public procurement cycles may pose challenges in non-university hospitals.
In Italy the industry is anticipated to grow at a CAGR of 5.9% from 2025 to 2035, reflecting slower uptake of anesthesia ultrasound systems compared to its EU neighbors. The country's procedural volumes are generally lower, and regional disparities in healthcare infrastructure slow nationwide adoption.
Nonetheless, outpatient surgery is on the rise, particularly in northern Italy, which is helping drive gradual awareness of UGRA’s advantages. Public sector investments remain modest, but there is growing traction in teaching hospitals and larger private surgical centres. To boost adoption, Italy may need more targeted physician training and funding incentives for high-precision anesthesia applications.
Sales in South Korea is anticipated to grow at a CAGR of 7.5% during the 2025 to 035 period, backed by strong private hospital investments and a national focus on surgical precision. The country is known for embracing fast-paced technological innovation, and its surgical centres are increasingly turning to advanced ultrasound-guided techniques for regional anesthesia.
The rising number of outpatient surgeries and cosmetic procedures, coupled with increased patient expectations for painless recovery, supports wider UGRA adoption. Government support for digital health and AI-integrated ultrasound technologies is also playing a role in industry expansion. Local manufacturers are beginning to compete with global OEMs on innovation.
Japan is anticipated to grow at a CAGR of 5.7% between 2025 and 2035, slightly under the global average, as hospitals maintain a conservative approach toward adopting new technologies. While the country has one of the oldest populations globally, leading to a rise in surgeries, there remains hesitancy in rapidly replacing existing anesthesia workflows.
Smaller hospitals, which make up a large part of Japan’s healthcare landscape, are often reluctant to invest in advanced systems due to perceived over-engineering. However, top-tier institutions and university hospitals are slowly driving adoption by integrating UGRA into orthopedic and pain management protocols.
Anesthesia ultra sound systems industry in China is anticipated to grow at a CAGR of 9.0% between 2025 and 2035, the highest among all countries, driven by rapid hospital modernization and expanding surgical capacity. The Chinese government is aggressively pushing for the adoption of advanced surgical technologies in Tier 1 and Tier 2 cities as part of its Healthy China 2030 initiative.
Rising healthcare expenditures and increased training in UGRA are fostering demand for anesthesia ultrasound systems. Domestic manufacturers are also emerging, making devices more affordable and accessible. Rural penetration remains limited but is expected to improve with government-led digital health outreach and mobile healthcare units.
Australia and New Zealand the sector are anticipated to grow at a CAGR of 6.6% over the 2025 to 2035 period, with moderate industry momentum supported by evolving surgical care models and high clinical training standards.
While the industry size is smaller relative to North America and Asia, both countries show consistent demand for UGRA, especially in orthopedic and trauma procedures. Public and private sector collaborations are fostering adoption of portable and AI-assisted ultrasound solutions. Remote areas benefit from mobile surgical units where anesthesia ultrasound is proving essential. Continued government funding and regulatory clarity will help sustain demand, especially in ambulatory and rural settings.
The anesthesia ultrasound systems industry is moderately consolidated, with a few dominant players such as GE Healthcare, Siemens Healthineers, Philips, Mindray, and Fujifilm SonoSite holding significant industry shares. These companies compete primarily through innovation, strategic partnerships, and global expansion to maintain their competitive edge.
Top companies are focusing on integrating artificial intelligence (AI) into their ultrasound systems to enhance diagnostic accuracy and operational efficiency. For instance, GE Healthcare's Venue Family point-of-care ultrasound systems now feature Caption Guidance, an AI-driven technology that provides real-time guidance for capturing diagnostic-quality cardiac images.
Similarly, Siemens Healthineers launched the ACUSON Maple ultrasound system, powered with AI to optimize everyday clinical performance for users of all skill levels. These innovations aim to improve image quality, streamline workflows, and support clinicians in various settings, including emergency departments and anesthesiology.
In February 2024, Shenzhen Mindray Bio-Medical Electronics Co., Ltd. launched its first handheld wireless ultrasound system, the TE Air, designed for seamless operation across various medical settings and offering high-clarity point-of-care ultrasound (POCUS) imaging.
In October 2024, GE Healthcare introduced the Venue Family point-of-care ultrasound systems, which incorporate Caption Guidance-an AI-based technology that delivers real-time assistance to clinicians for capturing diagnostic-quality cardiac images. Following that, in November 2024, Siemens Healthineers unveiled the ACUSON Maple ultrasound system, an AI-powered device engineered to enhance clinical performance across users of varying experience levels.
Philips Healthcare
Philips leads the anesthesia ultrasound systems industry with an estimated 21.2% share. The company's strength comes from its EPIQ and Affiniti ultrasound systems, which are widely used for nerve blocks and vascular access procedures. Philips has established a strong presence in North America and Europe through continuous innovation in needle guidance technology and partnerships with anesthesia training programs.
B. Braun Melsungen AG
B. Braun holds a significant position with an estimated 18.5% industry share. The company specializes in Stimuplex ultrasound-guided regional anesthesia solutions, including single-use nerve block needles. Its well-established distribution network in Germany, France, and the USA supports its leadership in clinical settings requiring precision anesthesia.
GE Healthcare
GE Healthcare accounts for approximately 15.8% of the industry. Its Venue and Logiq ultrasound systems are popular in anesthesia applications, particularly in emerging industries like India and China. GE's integration of AI-based tools for real-time needle tracking enhances its competitiveness.
SonoSite (Fujifilm)
SonoSite, now part of Fujifilm, holds around 12.3% of the industry. Known for its portable X-Porte and Edge II ultrasound systems, SonoSite dominates in ambulatory surgery centers and emergency anesthesia settings, especially in the USA and UK.
Mindray
Mindray captures about 9.7% of the industry, driven by its TE7 and M9 ultrasound systems. The company's cost-effective solutions have gained traction in China, Southeast Asia, and Africa, where budget-conscious healthcare providers seek reliable anesthesia imaging.
Esaote
Esaote holds a 6.5% industry share, with its MyLab ultrasound systems being widely adopted in Italy, Spain, and Latin America. The company focuses on musculoskeletal and nerve-specific imaging, making it a preferred choice for regional anesthesia.
Medovate
Medovate holds a 5.2% share, primarily due to its Safersonic hydrogel ultrasound coupling technology, which improves needle visibility during nerve blocks. The UK-based company has been expanding in Europe and Australia through collaborations with anesthesia specialists.
Alpinion Medical Systems
Alpinion holds around 4.8% of the industry, with its E-Cube series gaining popularity in South Korea and Japan. The company emphasizes high-resolution imaging for precise needle guidance in anesthesia procedures.
Telemed Ultrasound
Telemed holds a 3.6% share, focusing on compact, wireless ultrasound systems for anesthesia in remote and point-of-care settings. Its growth is strongest in Eastern Europe and parts of Asia.
Chison Medical Imaging
Chison holds approximately 3.1% of the industry, with its iVis and Eco series being used in China and other cost-sensitive regions. The company is gradually expanding into Europe through OEM partnerships.
Smiths Medical
Smiths Medical holds a 2.9% share, primarily due to its Portex nerve block needles and ultrasound-compatible accessories. Its presence in the USA and UK supports steady demand.
The industry is segmented into linear probe, curved array probe
The industry is divided into supraclavicular block, interscalene block, infraclavicular block, axillary block, femoral nerve block, transversus abdominis plane block
The industry is segmeted into hospital, ambulatory surgical centers
The industry is studied across North America, Latin America, Europe, South Asia, East Asia, Oceania, Middle East and Africa
The industry is expected to reach USD 723.3 million by 2035, growing at a CAGR of 7.4%.
Growth is driven by increased regional anesthesia procedures and adoption in hospitals and ASCs.
AI is enhancing real-time imaging and needle tracking, improving accuracy and expanding usage among generalists.
Asia-Pacific, especially India and China, is witnessing rapid growth due to affordable system deployment and public investment.
Focus on affordability, AI integration, and clinical training partnerships are critical for industry success.
Table 01: Global Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 02: Global Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 03: Global Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 04: Global Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 05: Global Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Region
Table 06: Global Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Region
Table 07: North America Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 08: North America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 09: North America Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 10: North America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 11: North America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 12: Latin America Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 13: Latin America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 14: Latin America Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 15: Latin America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 16: Latin America Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 17: Europe Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 18: Europe Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 19: Europe Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 20: Europe Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 21: Europe Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 22: South Asia Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 23: South Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 24: South Asia Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 25: South Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 26: South Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 27: East Asia Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 28: East Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 29: East Asia Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 30: East Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 31: East Asia Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 32: Oceania Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 33: Oceania Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 34: Oceania Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 35: Oceania Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 36: Oceania Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Table 37: Middle East and Africa Market Value (US$ Million) Analysis 2017 to 2022 and Forecast 2023 to 2033, By Country
Table 38: Middle East and Africa Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 39: Middle East and Africa Market Volume (Units) Analysis and Opportunity Assessment 2017 to 2033, By Product
Table 40: Middle East and Africa Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By Application
Table 41: Middle East and Africa Market Value (US$ Million) Analysis and Opportunity Assessment 2017 to 2033, By End User
Figure 01: Global Market Volume (Units), 2017 to 2022
Figure 02: Global Market Volume (Units) and Y-o-Y Growth (%) Analysis, 2023 to 2033
Figure 03: Global Market, Pricing Analysis per unit (US$), in 2023
Figure 04: Global Market, Pricing Forecast per unit (US$), in 2033
Figure 05: Global Market Value (US$ Million) Analysis, 2017 to 2022
Figure 06: Global Market Forecast and Y-o-Y Growth, 2023 to 2033
Figure 07: Global Market Absolute $ Opportunity (US$ Million) Analysis, 2023
Figure 08: Global Market Value Share (%) Analysis 2023 and 2033, By Product
Figure 09: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By Product
Figure 10: Global Market Attractiveness Analysis 2023 to 2033, By Product
Figure 11: Global Market Value Share (%) Analysis 2023 and 2033, By Application
Figure 12: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By Application
Figure 13: Global Market Attractiveness Analysis 2023 to 2033, By Application
Figure 14: Global Market Value Share (%) Analysis 2023 and 2033, By End User
Figure 15: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By End User
Figure 16: Global Market Attractiveness Analysis 2023 to 2033, By End User
Figure 17: Global Market Value Share (%) Analysis 2023 and 2033, By Region
Figure 18: Global Market Y-o-Y Growth (%) Analysis 2023 to 2033, By Region
Figure 19: Global Market Attractiveness Analysis 2023 to 2033, By Region
Figure 20: North America Market Value (US$ Million) Analysis, 2017 to 2022
Figure 21: North America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 22: North America Market Value Share, By Product (2023 E)
Figure 23: North America Market Value Share, By Application (2023 E)
Figure 24: North America Market Value Share, By End User (2023 E)
Figure 25: North America Market Value Share, by Country (2023 E)
Figure 26: North America Market Attractiveness Analysis By Product, 2023 to 2033
Figure 27: North America Market Attractiveness Analysis By Application, 2023 to 2033
Figure 28: North America Market Attractiveness Analysis By End User, 2023 to 2033
Figure 29: North America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 30: United States Market Value Proportion Analysis, 2022
Figure 31: Global Vs. United States Growth Comparison
Figure 32: United States Market Share Analysis (%) By Product, 2023 to 2033
Figure 33: United States Market Share Analysis (%) By Application, 2023 to 2033
Figure 34: United States Market Share Analysis (%) By End User, 2023 to 2033
Figure 35: Canada Market Value Proportion Analysis, 2022
Figure 36: Global Vs. Canada. Growth Comparison
Figure 37: Canada Market Share Analysis (%) By Product, 2023 to 2033
Figure 38: Canada Market Share Analysis (%) By Application, 2023 to 2033
Figure 39: Canada Market Share Analysis (%) By End User, 2023 to 2033
Figure 40: Latin America Market Value (US$ Million) Analysis, 2017 to 2022
Figure 41: Latin America Market Value (US$ Million) Forecast, 2023 to 2033
Figure 42: Latin America Market Value Share, By Product (2023 E)
Figure 43: Latin America Market Value Share, By Application (2023 E)
Figure 44: Latin America Market Value Share, By End User (2023 E)
Figure 45: Latin America Market Value Share, by Country (2023 E)
Figure 46: Latin America Market Attractiveness Analysis By Product, 2023 to 2033
Figure 47: Latin America Market Attractiveness Analysis By Application, 2023 to 2033
Figure 48: Latin America Market Attractiveness Analysis By End User, 2023 to 2033
Figure 49: Latin America Market Attractiveness Analysis by Country, 2023 to 2033
Figure 50: Mexico Market Value Proportion Analysis, 2022
Figure 51: Global Vs Mexico Growth Comparison
Figure 52: Mexico Market Share Analysis (%) By Product, 2023 to 2033
Figure 53: Mexico Market Share Analysis (%) By Application, 2023 to 2033
Figure 54: Mexico Market Share Analysis (%) By End User, 2023 to 2033
Figure 55: Brazil Market Value Proportion Analysis, 2022
Figure 56: Global Vs. Brazil. Growth Comparison
Figure 57: Brazil Market Share Analysis (%) By Product, 2023 to 2033
Figure 58: Brazil Market Share Analysis (%) By Application, 2023 to 2033
Figure 59: Brazil Market Share Analysis (%) By End User, 2023 to 2033
Figure 60: Argentina Market Value Proportion Analysis, 2022
Figure 61: Global Vs Argentina Growth Comparison
Figure 62: Argentina Market Share Analysis (%) By Product, 2023 to 2033
Figure 63: Argentina Market Share Analysis (%) By Application, 2023 to 2033
Figure 64: Argentina Market Share Analysis (%) By End User, 2023 to 2033
Figure 65: Europe Market Value (US$ Million) Analysis, 2017 to 2022
Figure 66: Europe Market Value (US$ Million) Forecast, 2023 to 2033
Figure 67: Europe Market Value Share, By Product (2023 E)
Figure 68: Europe Market Value Share, By Application (2023 E)
Figure 69: Europe Market Value Share, By End User (2023 E)
Figure 70: Europe Market Value Share, by Country (2023 E)
Figure 71: Europe Market Attractiveness Analysis By Product, 2023 to 2033
Figure 72: Europe Market Attractiveness Analysis By Application, 2023 to 2033
Figure 73: Europe Market Attractiveness Analysis By End User, 2023 to 2033
Figure 74: Europe Market Attractiveness Analysis by Country, 2023 to 2033
Figure 75: United Kingdom Market Value Proportion Analysis, 2022
Figure 76: Global Vs. United Kingdom Growth Comparison
Figure 77: United Kingdom Market Share Analysis (%) By Product, 2023 to 2033
Figure 78: United Kingdom Market Share Analysis (%) By Application, 2023 to 2033
Figure 79: United Kingdom Market Share Analysis (%) By End User, 2023 to 2033
Figure 80: Germany Market Value Proportion Analysis, 2022
Figure 81: Global Vs. Germany Growth Comparison
Figure 82: Germany Market Share Analysis (%) By Product, 2023 to 2033
Figure 83: Germany Market Share Analysis (%) By Application, 2023 to 2033
Figure 84: Germany Market Share Analysis (%) By End User, 2023 to 2033
Figure 85: Italy Market Value Proportion Analysis, 2022
Figure 86: Global Vs. Italy Growth Comparison
Figure 87: Italy Market Share Analysis (%) By Product, 2023 to 2033
Figure 88: Italy Market Share Analysis (%) By Application, 2023 to 2033
Figure 89: Italy Market Share Analysis (%) By End User, 2023 to 2033
Figure 90: France Market Value Proportion Analysis, 2022
Figure 91: Global Vs France Growth Comparison
Figure 92: France Market Share Analysis (%) By Product, 2023 to 2033
Figure 93: France Market Share Analysis (%) By Application, 2023 to 2033
Figure 94: France Market Share Analysis (%) By End User, 2023 to 2033
Figure 95: Spain Market Value Proportion Analysis, 2022
Figure 96: Global Vs Spain Growth Comparison
Figure 97: Spain Market Share Analysis (%) By Product, 2023 to 2033
Figure 98: Spain Market Share Analysis (%) By Application, 2023 to 2033
Figure 99: Spain Market Share Analysis (%) By End User, 2023 to 2033
Figure 100: Russia Market Value Proportion Analysis, 2022
Figure 101: Global Vs Russia Growth Comparison
Figure 102: Russia Market Share Analysis (%) By Product, 2023 to 2033
Figure 103: Russia Market Share Analysis (%) By Application, 2023 to 2033
Figure 104: Russia Market Share Analysis (%) By End User, 2023 to 2033
Figure 105: BENELUX Market Value Proportion Analysis, 2022
Figure 106: Global Vs BENELUX Growth Comparison
Figure 107: BENELUX Market Share Analysis (%) By Product, 2023 to 2033
Figure 108: BENELUX Market Share Analysis (%) By Application, 2023 to 2033
Figure 109: BENELUX Market Share Analysis (%) By End User, 2023 to 2033
Figure 110: East Asia Market Value (US$ Million) Analysis, 2017 to 2022
Figure 111: East Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 112: East Asia Market Value Share, By Product (2023 E)
Figure 113: East Asia Market Value Share, By Application (2023 E)
Figure 114: East Asia Market Value Share, By End User (2023 E)
Figure 115: East Asia Market Value Share, by Country (2023 E)
Figure 116: East Asia Market Attractiveness Analysis By Product, 2023 to 2033
Figure 117: East Asia Market Attractiveness Analysis By Application, 2023 to 2033
Figure 118: East Asia Market Attractiveness Analysis By End User, 2023 to 2033
Figure 119: East Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 120: China Market Value Proportion Analysis, 2022
Figure 121: Global Vs. China Growth Comparison
Figure 122: China Market Share Analysis (%) By Product, 2023 to 2033
Figure 123: China Market Share Analysis (%) By Application, 2023 to 2033
Figure 124: China Market Share Analysis (%) By End User, 2023 to 2033
Figure 125: Japan Market Value Proportion Analysis, 2022
Figure 126: Global Vs. Japan Growth Comparison
Figure 127: Japan Market Share Analysis (%) By Product, 2023 to 2033
Figure 128: Japan Market Share Analysis (%) By Application, 2023 to 2033
Figure 129: Japan Market Share Analysis (%) By End User, 2023 to 2033
Figure 130: South Korea Market Value Proportion Analysis, 2022
Figure 131: Global Vs South Korea Growth Comparison
Figure 132: South Korea Market Share Analysis (%) By Product, 2023 to 2033
Figure 133: South Korea Market Share Analysis (%) By Application, 2023 to 2033
Figure 134: South Korea Market Share Analysis (%) By End User, 2023 to 2033
Figure 135: South Asia Market Value (US$ Million) Analysis, 2017 to 2022
Figure 136: South Asia Market Value (US$ Million) Forecast, 2023 to 2033
Figure 137: South Asia Market Value Share, By Product (2023 E)
Figure 138: South Asia Market Value Share, By Application (2023 E)
Figure 139: South Asia Market Value Share, By End User (2023 E)
Figure 140: South Asia Market Value Share, by Country (2023 E)
Figure 141: South Asia Market Attractiveness Analysis By Product, 2023 to 2033
Figure 142: South Asia Market Attractiveness Analysis By Application, 2023 to 2033
Figure 143: South Asia Market Attractiveness Analysis By End User, 2023 to 2033
Figure 144: South Asia Market Attractiveness Analysis by Country, 2023 to 2033
Figure 145: India Market Value Proportion Analysis, 2022
Figure 146: Global Vs. India Growth Comparison
Figure 147: India Market Share Analysis (%) By Product, 2023 to 2033
Figure 148: India Market Share Analysis (%) By Application, 2023 to 2033
Figure 149: India Market Share Analysis (%) By End User, 2023 to 2033
Figure 150: Indonesia Market Value Proportion Analysis, 2022
Figure 151: Global Vs. Indonesia Growth Comparison
Figure 152: Indonesia Market Share Analysis (%) By Product, 2023 to 2033
Figure 153: Indonesia Market Share Analysis (%) By Application, 2023 to 2033
Figure 154: Indonesia Market Share Analysis (%) By End User, 2023 to 2033
Figure 155: Malaysia Market Value Proportion Analysis, 2022
Figure 156: Global Vs. Malaysia Growth Comparison
Figure 157: Malaysia Market Share Analysis (%) By Product, 2023 to 2033
Figure 158: Malaysia Market Share Analysis (%) By Application, 2023 to 2033
Figure 159: Malaysia Market Share Analysis (%) By End User, 2023 to 2033
Figure 160: Thailand Market Value Proportion Analysis, 2022
Figure 161: Global Vs. Thailand Growth Comparison
Figure 162: Thailand Market Share Analysis (%) By Product, 2023 to 2033
Figure 163: Thailand Market Share Analysis (%) By Application, 2023 to 2033
Figure 164: Thailand Market Share Analysis (%) By End User, 2023 to 2033
Figure 165: Oceania Market Value (US$ Million) Analysis, 2017 to 2022
Figure 166: Oceania Market Value (US$ Million) Forecast, 2023 to 2033
Figure 167: Oceania Market Value Share, By Product (2023 E)
Figure 168: Oceania Market Value Share, By Application (2023 E)
Figure 169: Oceania Market Value Share, By End User (2023 E)
Figure 170: Oceania Market Value Share, by Country (2023 E)
Figure 171: Oceania Market Attractiveness Analysis By Product, 2023 to 2033
Figure 172: Oceania Market Attractiveness Analysis By Application, 2023 to 2033
Figure 173: Oceania Market Attractiveness Analysis By End User, 2023 to 2033
Figure 174: Oceania Market Attractiveness Analysis by Country, 2023 to 2033
Figure 175: Australia Market Value Proportion Analysis, 2022
Figure 176: Global Vs. Australia Growth Comparison
Figure 177: Australia Market Share Analysis (%) By Product, 2023 to 2033
Figure 178: Australia Market Share Analysis (%) By Application, 2023 to 2033
Figure 179: Australia Market Share Analysis (%) By End User, 2023 to 2033
Figure 180: New Zealand Market Value Proportion Analysis, 2022
Figure 181: Global Vs New Zealand Growth Comparison
Figure 182: New Zealand Market Share Analysis (%) By Product, 2023 to 2033
Figure 183: New Zealand Market Share Analysis (%) By Application, 2023 to 2033
Figure 184: New Zealand Market Share Analysis (%) By End User, 2023 to 2033
Figure 185: Middle East and Africa Market Value (US$ Million) Analysis, 2017 to 2022
Figure 186: Middle East and Africa Market Value (US$ Million) Forecast, 2023 to 2033
Figure 187: Middle East and Africa Market Value Share, By Product (2023 E)
Figure 188: Middle East and Africa Market Value Share, By Application (2023 E)
Figure 189: Middle East and Africa Market Value Share, By End User (2023 E)
Figure 190: Middle East and Africa Market Value Share, by Country (2023 E)
Figure 191: Middle East and Africa Market Attractiveness Analysis By Product, 2023 to 2033
Figure 192: Middle East and Africa Market Attractiveness Analysis By Application, 2023 to 2033
Figure 193: Middle East and Africa Market Attractiveness Analysis By End User, 2023 to 2033
Figure 194: Middle East and Africa Market Attractiveness Analysis by Country, 2023 to 2033
Figure 195: GCC Countries Market Value Proportion Analysis, 2022
Figure 196: Global Vs GCC Countries Growth Comparison
Figure 197: GCC Countries Market Share Analysis (%) By Product, 2023 to 2033
Figure 198: GCC Countries Market Share Analysis (%) By Application, 2023 to 2033
Figure 199: GCC Countries Market Share Analysis (%) By End User, 2023 to 2033
Figure 200: Türkiye Market Value Proportion Analysis, 2022
Figure 201: Global Vs. Türkiye Growth Comparison
Figure 202: Türkiye Market Share Analysis (%) By Product, 2023 to 2033
Figure 203: Türkiye Market Share Analysis (%) By Application, 2023 to 2033
Figure 204: Türkiye Market Share Analysis (%) By End User, 2023 to 2033
Figure 205: South Africa Market Value Proportion Analysis, 2022
Figure 206: Global Vs. South Africa Growth Comparison
Figure 207: South Africa Market Share Analysis (%) By Product, 2023 to 2033
Figure 208: South Africa Market Share Analysis (%) By Application, 2023 to 2033
Figure 209: South Africa Market Share Analysis (%) By End User, 2023 to 2033
Figure 210: Northern Africa Market Value Proportion Analysis, 2022
Figure 211: Global Vs Northern Africa Growth Comparison
Figure 212: Northern Africa Market Share Analysis (%) By Product, 2023 to 2033
Figure 213: Northern Africa Market Share Analysis (%) By Application, 2023 to 2033
Figure 214: Northern Africa Market Share Analysis (%) By End User, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Anesthesia Breathing Bags Market Size and Share Forecast Outlook 2025 to 2035
Anesthesia Equipment Market Size and Share Forecast Outlook 2025 to 2035
Anesthesia Machines Market - Size, Share, and Forecast 2025-2035
Dental Anesthesia Delivery Systems Market
General Anesthesia Drugs Market Insights – Trends & Forecast 2025 to 2035
Portable Anesthesia Systems Market Growth - Trends & Forecast 2025 to 2035
Veterinary Anesthesia Machines Market Size and Share Forecast Outlook 2025 to 2035
Mobile Animal Inhalation Anesthesia Machine Market Size and Share Forecast Outlook 2025 to 2035
Ultrasound Skin Tightening Devices Market Size and Share Forecast Outlook 2025 to 2035
Ultrasound Biometry Devices Market Size and Share Forecast Outlook 2025 to 2035
Ultrasound Devices Market Size and Share Forecast Outlook 2025 to 2035
Ultrasound-Guided Breast Biopsy Market Size and Share Forecast Outlook 2025 to 2035
Global Ultrasound Conductivity Gels Market Insights – Size, Share & Industry Growth 2025–2035
Global Ultrasound Market Insights – Trends & Forecast 2024-2034
Ultrasound Imaging Solution Market
Ultrasound Systems Market Growth – Trends & Forecast 2025-2035
Teleultrasound Systems Market
Food Ultrasound Market Analysis – Applications & Innovations 2025 to 2035
Micro-Ultrasound Systems Market
Focused Ultrasound System Market Trends and Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA