The arthralgia management market is valued at USD 7.72 billion in 2025. As per FMI's analysis, the arthralgia management market will grow at a CAGR of 9.1% and reach USD 18.46 billion by 2035.
In 2024, the industry experienced steady growth driven by increasing incidences of arthritis, osteoarthritis, and musculoskeletal disorders. Advances in pain management therapies, including nonsteroidal anti-inflammatory drugs (NSAIDs), biologics, and corticosteroids, contributed significantly to industry expansion.
Additionally, the adoption of personalized medicine and targeted drug delivery systems gained traction, enhancing treatment efficacy and reducing side effects. The integration of telehealth services also allowed for wider accessibility to consultations and chronic pain management, especially in rural regions.
On the regulatory front, approvals for innovative therapeutics and faster clinical trial processes have accelerated product launches. Collaborations between pharmaceutical companies and research institutions expanded the pipeline for novel drug candidates.
Furthermore, growing patient awareness and proactive management of joint pain fostered increased adoption of both pharmaceutical and non-pharmaceutical solutions.
Looking ahead in 2025, the industry is expected to witness further advancements in regenerative medicine, including stem cell therapy and gene therapy. Companies will likely invest in AI-powered diagnostic tools for early detection of joint disorders.
The rising geriatric population and higher healthcare expenditure in developing regions will act as key growth drivers. Additionally, government initiatives supporting pain management programs will further propel industry growth.
Market Value Insights
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 7.72 billion |
| Industry Value (2035F) | USD 18.46 billion |
| CAGR (2025 to 2035) | 9.1% |
The Arthralgia Management Industry is expected to grow steadily, driven by the rising prevalence of joint-related disorders and pain management therapy advancements. Pharmaceutical companies, biotech firms, and healthcare providers stand to benefit from increasing demand for innovative treatments and personalized care solutions. However, companies with outdated or ineffective therapies may struggle to compete in this rapidly evolving landscape.
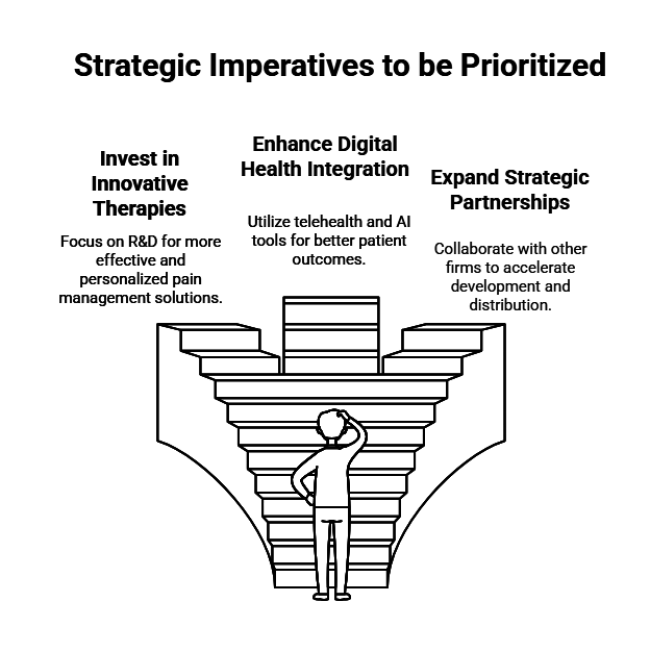
Invest in Innovative Therapies: Pharmaceutical companies should prioritize R&D investments in biologics, gene therapies, and regenerative medicine to offer more effective and personalized pain management solutions.
Enhance Digital Health Integration: Leverage telehealth platforms and AI-powered diagnostic tools to improve early detection, patient monitoring, and remote pain management, ensuring greater accessibility and better outcomes.
Expand Strategic Partnerships: Form alliances with research institutions, biotechnology firms, and digital health companies to accelerate product development, optimize distribution channels, and enhance industry penetration globally.
| Risk | Probability & Impact |
|---|---|
| Regulatory Delays | High Probability, High Impact |
| Industry Competition | Medium Probability, High Impact |
| Supply Chain Disruptions | Medium Probability, Moderate Impact |
1-year Executive Watchlist
| Priority | Immediate Action |
|---|---|
| Enhance Technological Capabilities | Invest in R&D for AI-assisted diagnostics and minimally invasive procedures. |
| Expand Industry Access | Form partnerships with healthcare providers in emerging industries. |
| Strengthen Supply Chain | Establish reliable supplier networks and monitor regulatory changes. |
To stay ahead, companies must prioritize investment in cutting-edge arthralgia management therapies, particularly those leveraging AI, biologics, and regenerative medicine.
These technological advancements will not only differentiate their offerings in a competitive industry but also improve patient outcomes through more personalized treatment options.
Strategic partnerships with healthcare providers, digital health platforms, and research institutions will be key in expanding industry access and accelerating innovation.
Furthermore, strengthening the supply chain by establishing reliable networks and monitoring regulatory changes will ensure sustained growth. By realigning their roadmap to focus on these areas, companies can position themselves as leaders in the rapidly evolving arthralgia management space, ultimately capturing a larger share of the industry by 2035.
Surveyed Q4 2024, n=450 stakeholder participants evenly distributed across pharmaceutical companies, healthcare providers, distributors, and patients in the USA, Western Europe, Japan, and South Korea
Regional Variance
High Variance
Convergent and Divergent Perspectives on ROI
74% of USA stakeholders determined advanced treatments like biologics to be “worth the investment” while 45% in Japan still rely on traditional methods for joint pain management.
Consensus
Biologics: Chosen by 68% globally due to their potential for more targeted and long-lasting pain relief.
Variance
Shared Challenges
84% cited rising treatment costs (e.g., biologics, advanced diagnostics) as a primary concern.
Regional Differences
Manufacturers
Distributors
End-Users (Patients/Healthcare Providers)
Alignment
71% of global pharmaceutical companies plan to increase investments in biologics and regenerative medicine R&D.
Divergence
USA
75% cited the growing complexity of healthcare regulations (e.g., FDA approvals) as a major disruptor to fast-tracking new treatments.
Western Europe
82% viewed stricter EU regulations on biologics as a potential growth driver for high-quality, differentiated products.
Japan/South Korea
43% believed regulatory frameworks were easing, leading to faster approval for innovative pain management solutions.
High Consensus: Regulatory compliance, patient safety, and cost pressures are global concerns.
Key Variances
Strategic Insight: A one-size-fits-all approach will not succeed in the arthralgia management industry. Regional customization, such as biologic treatments in the USA, regenerative solutions in Europe, and personalized care in Asia, is essential to maximize growth and industry penetration.
| Countries | Impact of Policies, Government Regulations, and Mandatory Certifications |
|---|---|
| United States | FDA regulat es biologics, requiring clinical trials for approval. Companies must comply with FDA, GMP, and HIPAA for pati ent data privacy. |
| United Kingdom | MHRA oversees medications and biologics. CE marking is required for medical devices. UK Medici nes and Medical Devices Act 2021 strengthens regulation post-Brexit. |
| France | ANSM ensures safety of medications and biologics. CE mark is required for medical devices. Products must be registered with ANSM and meet AFNOR certification. |
| Germany | BfArM regulates biologics and medications. CE marking and GMP certification are required for devices and drug manufacturing. |
| Italy | AIFA regulates pharmaceutical products. CE marking is needed for medical devices, AIF A registration for biologics, and GMP certification for manufacturers. |
| South Korea | MFDS regulates medicines and biologics. Ap proval by MFDS and GMP certification are mandatory, with KFDA approval for devices. |
| Japan | PMDA oversees drug and biologic approvals. PMDA approval is required for biologics, and devices must meet JIS (Japanese Industrial Standards) certification. |
| China | NMPA regulates drugs, biologics, and medical devices. Stringent approval processes and local clinical trials are required for new biologics. |
| Australia-NZ | TGA regulates biologics and medications. Companies must comply with TGA and GMP for manufacturing. CE marking may be required for devices. |
The drug class segment is expected to experience substantial growth due to increasing advancements in biologics and pain management therapies. According to FMI, the global CAGR for this segment is approximately 9.0% from 2025 to 2035. The growing demand for disease-modifying anti-rheumatic drugs (DMARDs), biologics, and corticosteroids are driving this expansion.
These treatments are highly effective in managing conditions like rheumatoid arthritis and osteoarthritis. Additionally, newer treatments, such as gene therapies and regenerative medicine, are expected to contribute significantly to the growth of this segment. The availability of these advanced therapies has bolstered the demand for pain management solutions in arthralgia, particularly in aging populations.
The application segment, driven by conditions such as osteoarthritis, rheumatoid arthritis, and other joint-related diseases, is expected to grow at a 9.2% CAGR. This segment is benefiting from the growing awareness of joint health and the increasing prevalence of osteoarthritis, especially in older populations globally.
With rising health concerns and better diagnostics, more patients are opting for early interventions, contributing to the demand for effective therapeutics. Moreover, advancements in treatment approaches, such as personalized pain management, are further boosting the growth prospects.
FMI opines that this segment has significant growth potential, particularly in emerging industries, where arthritis prevalence is increasing.
The online channel segment is also expected to grow at a 9.0% CAGR. This growth is largely attributed to the expanding role of online pharmacies and retail healthcare outlets in making pain management solutions more accessible.
The increasing shift toward digital health platforms and telemedicine services is enabling easier access to pain therapeutics, especially in regions with a high number of elderly individuals who suffer from joint pain.
Additionally, e-commerce platforms have made it more convenient for consumers to purchase over-the-counter and prescription-based pain management drugs.
As per FMI’s analysis, this trend is expected to continue as digital healthcare infrastructure grows and consumers demand more convenience in accessing treatments.
United States’ Arthralgia Management industry is anticipated to grow at 9.3% CAGR, due to the strong healthcare infrastructure, increased adoption of advanced pain management therapies, and the increasing aging population.
The USA remains one of the largest industries, and there is significant need for new therapies for arthralgia, particularly biologics and regenerative treatments.
Growth is also supported by government regulations and healthcare policies, such as insurance coverage for arthritis treatments.
The increasing application of telemedicine for pain management and innovations in diagnostic technologies like AI and IoT devices contribute to this industry growth, making the USA a major player in the Arthralgia Management industry.
United Kingdom’s Arthralgia Management industry is anticipated to grow at 8.8% CAGR. United Kingdom industry is primarily driven by rising government expenditure on healthcare and research in the country and increasing the number of arthritis-related diseases.
The UK government's focus on improving access to health care and funding for biologics will be powerful forces propelling the industry forward.
Some key developments include initiatives from the National Health Service (NHS) to improve access to therapies, and a trend towards personalization in medicine.
According to FMI, increasing adoption of technologies including AIin diagnostics and treatment planning is expected to further accelerate industry growth in the UK.
France’s Arthralgia Management industry is anticipated to grow at 9.0% CAGR. The country benefits from strong public healthcare systems and a growing emphasis on innovative treatments for arthralgia.
France's adoption of biologics and personalized medicine is set to increase as patients seek more effective treatments.
The country is witnessing a rise in the elderly population, which drives demand for advanced pain management solutions. Government funding for R&D in healthcare and regulations that support drug approvals will continue to foster industry growth.
As a leading healthcare industry in Europe, France is poised to experience significant growth in the Arthralgia Management industry.
Germany’s Arthralgia Management industry is anticipated to grow at 9.2% CAGR. This growth is aided by Germany’s robust healthcare system, established pharmaceutical industry, and growing elderly demographic.
The industry for treatment of arthralgia is growing, with a trend towards the biologics and regenerative medicine.
Germany's high investment in healthcare infrastructure and research will keep it a significant player in the Arthralgia Management industry.
Moreover, factors such as regulatory support in the form of fast-track approvals for new treatments, and increased emphasis on personalized medicine, are expected to drive industry growth in Germany in the coming decade.
Italy’s Arthralgia Management industry is anticipated to rise at 8.5% CAGR. Increasing demand for effective management for arthritis pain, especially biologics and new generation treatments, will drive the Arthralgia Management industry in the Italy. Italy’s public healthcare system is still evolving, but it is gradually improving access to more advanced therapies.
FMI opines that the significant rise in the aging population is anticipated to create surge in healthcare expenditure and increase the demand for arthralgia treatments.
Government regulations surrounding pharmaceuticals, as well as growing attention to personalized healthcare, are also expected to drive the industry.
South Korea’s Arthralgia Management industry is anticipated to grow at a CAGR of 9.0% during the forecast period. The country will continue to witness growth in its Arthralgia Management industry, as the elderly population is on the rise, and there is demand for advanced, effective pain management therapies.
The country has grown significantly in the field of medical technology, and the innovative features such as AI-based diagnostics and telemedicine are drastically changing the treatment models.
South Korea's government is pouring capital into improving its healthcare capabilities which should enable access to and adoption of novel biologic therapies.
The industry is likely to grow significantly in South Korea due to its tech-savvy people and growing focus on healthcare.
Japan’s Arthralgia Management industry is expected to have growth at 8.2% CAGR. One of the major factor of the Arthralgia Management industry in Japan is the aging population of the country, as more and more individuals develop arthritis and other joint-related ailments. Robust healthcare infrastructure in Japan is likely to aid the gradual growth of the pain management therapeutics industry.
Additionally, the emergence of advanced pain management therapies in the industry will contribute to this growth. The increased availability of healthcare and funding for arthritis treatments through the Japanese government will also continue to drive demand for novel arthralgia therapeutics over the next decade.
ChinaChina’s Arthralgia Management industry is anticipated to grow at 9.5% CAGR. China’s rapidly expanding healthcare infrastructure, increasing healthcare spending, and rising elderly population contribute to a strong demand for arthralgia treatments. The adoption of advanced pain management therapies, including biologics and gene therapies, is growing, driven by both government investments and the expansion of private healthcare services.
FMI opines that the Arthralgia Management industry is expected to expand significantly as the country shifts toward more personalized medicine China’s regulatory environment has become more favourable for pharmaceutical innovation, creating a favourable industry landscape for the next decade.
The industry in Australia-NZ is expected to grow at 8.9% CAGR The healthcare systems of Australia and New Zealand are developing and expanding with more government expenditure on healthcare and increasing utilisation of arthralgia treatments, particularly advanced therapies, are expected to grow during this period.
Both nations have a growing ageing population, resulting in a higher need for new pain management treatments.
These advancements in the digital health ecosystem, with significant progress in areas such as telemedicine, AI-powered diagnostics, and wearables, especially notable in Australia.
Healthcare providers will continue to integrate new therapies, and steady yearly growth can be anticipated while growing economic restraints gain traction.
In 2024, several key players in the Arthralgia Management industry have made significant advancements. Pfizer Inc. successfully completed late-stage clinical trials for a new biologic treatment aimed at alleviating joint pain caused by osteoarthritis.
The treatment showed promising results in reducing inflammation and pain severity, greatly improving the quality of life for patients with chronic conditions.
Johnson & Johnson received FDA approval for a new injectable formulation of its anti-inflammatory drug designed for patients with severe arthralgia pain.
This new formulation reduces the frequency of administration, making it more convenient for patients to manage their symptoms. Bristol Myers Squibb expanded its portfolio through the acquisition of a small biotechnology firm specializing in gene therapies for rheumatoid arthritis, aiming to enhance its biologics and gene therapy offerings.
AbbVie launched a new oral treatment for osteoarthritis patients in early 2024, offering relief from joint pain without the common side effects of traditional NSAIDs.
Industry feedback indicates positive outlook, with expectations for strong growth in both the USA and European industries. Merck & Co. announced a collaboration with a digital health company to create a mobile platform that tracks osteoarthritis symptoms and pain levels, aimed at improving patient outcomes by providing real-time data to both patients and healthcare providers.
Industry Share Analysis
Pfizer Inc.
Novartis AG
Johnson & Johnson
GlaxoSmithKline plc
Sanofi
Boehringer Ingelheim
AstraZeneca
Merck & Co., Inc.
Teva Pharmaceutical Industries Ltd.
Bristol-Myers Squibb Company
F. Hoffmann-La Roche Ltd.
Viatris (ex-Mylan N.V.)
The industry is divided into NSAID, corticosteroids, antidepressants, anticonvulsants, antibiotics
The industry is segmented into knee and ankle pain, hip pain, shoulder and elbow pain, and others.
The industry is segmented into hospital pharmacies, retail pharmacies, and online pharmacies.
The industry is studied across North America, Latin America, Europe, Asia Pacific, and Middle East & Africa.
The market is expected to grow from USD 7.72 billion in 2025 to USD 18.46 billion by 2035, at a CAGR of 9.1%.
Key risks for the stakeholders include regulatory delays (high probability and impact), industry competition (medium probability and high impact), and supply chain disruptions (medium probability and moderate impact).
The industry’s growth is driven by biologics, disease-modifying anti-rheumatic drugs (DMARDs), corticosteroids, and newer treatments like gene therapies and regenerative medicine.
The growth is driven by rising joint health awareness, the increasing prevalence of osteoarthritis, and advancements in personalized pain management.
The expansion is fueled by the rise of online pharmacies, e-commerce platforms, and digital health services, making pain management solutions more accessible.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2018 to 2033
Table 2: Global Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 3: Global Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 4: Global Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 5: North America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 6: North America Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 7: North America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 8: North America Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 9: Latin America Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 10: Latin America Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 11: Latin America Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 12: Latin America Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 13: Europe Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 14: Europe Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 15: Europe Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 16: Europe Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 17: Asia Pacific Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 18: Asia Pacific Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 19: Asia Pacific Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 20: Asia Pacific Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Table 21: Middle East and Africa Market Value (US$ Million) Forecast by Country, 2018 to 2033
Table 22: Middle East and Africa Market Value (US$ Million) Forecast by Drug Class, 2018 to 2033
Table 23: Middle East and Africa Market Value (US$ Million) Forecast by Application, 2018 to 2033
Table 24: Middle East and Africa Market Value (US$ Million) Forecast by Distribution Channel , 2018 to 2033
Figure 1: Global Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 2: Global Market Value (US$ Million) by Application, 2023 to 2033
Figure 3: Global Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 4: Global Market Value (US$ Million) by Region, 2023 to 2033
Figure 5: Global Market Value (US$ Million) Analysis by Region, 2018 to 2033
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2023 to 2033
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2023 to 2033
Figure 8: Global Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 9: Global Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 10: Global Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 11: Global Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 12: Global Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 13: Global Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 14: Global Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 15: Global Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 16: Global Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 17: Global Market Attractiveness by Drug Class, 2023 to 2033
Figure 18: Global Market Attractiveness by Application, 2023 to 2033
Figure 19: Global Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 20: Global Market Attractiveness by Region, 2023 to 2033
Figure 21: North America Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 22: North America Market Value (US$ Million) by Application, 2023 to 2033
Figure 23: North America Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 24: North America Market Value (US$ Million) by Country, 2023 to 2033
Figure 25: North America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 26: North America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 27: North America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 28: North America Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 29: North America Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 30: North America Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 31: North America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 32: North America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 33: North America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 34: North America Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 35: North America Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 36: North America Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 37: North America Market Attractiveness by Drug Class, 2023 to 2033
Figure 38: North America Market Attractiveness by Application, 2023 to 2033
Figure 39: North America Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 40: North America Market Attractiveness by Country, 2023 to 2033
Figure 41: Latin America Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 42: Latin America Market Value (US$ Million) by Application, 2023 to 2033
Figure 43: Latin America Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 44: Latin America Market Value (US$ Million) by Country, 2023 to 2033
Figure 45: Latin America Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 48: Latin America Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 49: Latin America Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 50: Latin America Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 51: Latin America Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 52: Latin America Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 53: Latin America Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 54: Latin America Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 55: Latin America Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 56: Latin America Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 57: Latin America Market Attractiveness by Drug Class, 2023 to 2033
Figure 58: Latin America Market Attractiveness by Application, 2023 to 2033
Figure 59: Latin America Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 60: Latin America Market Attractiveness by Country, 2023 to 2033
Figure 61: Europe Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 62: Europe Market Value (US$ Million) by Application, 2023 to 2033
Figure 63: Europe Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 64: Europe Market Value (US$ Million) by Country, 2023 to 2033
Figure 65: Europe Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 66: Europe Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 67: Europe Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 68: Europe Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 69: Europe Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 70: Europe Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 71: Europe Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 72: Europe Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 73: Europe Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 74: Europe Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 75: Europe Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 76: Europe Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 77: Europe Market Attractiveness by Drug Class, 2023 to 2033
Figure 78: Europe Market Attractiveness by Application, 2023 to 2033
Figure 79: Europe Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 80: Europe Market Attractiveness by Country, 2023 to 2033
Figure 81: Asia Pacific Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 82: Asia Pacific Market Value (US$ Million) by Application, 2023 to 2033
Figure 83: Asia Pacific Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 84: Asia Pacific Market Value (US$ Million) by Country, 2023 to 2033
Figure 85: Asia Pacific Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 86: Asia Pacific Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 87: Asia Pacific Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 88: Asia Pacific Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 89: Asia Pacific Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 90: Asia Pacific Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 91: Asia Pacific Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 92: Asia Pacific Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 93: Asia Pacific Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 94: Asia Pacific Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 95: Asia Pacific Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 96: Asia Pacific Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 97: Asia Pacific Market Attractiveness by Drug Class, 2023 to 2033
Figure 98: Asia Pacific Market Attractiveness by Application, 2023 to 2033
Figure 99: Asia Pacific Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 100: Asia Pacific Market Attractiveness by Country, 2023 to 2033
Figure 101: Middle East and Africa Market Value (US$ Million) by Drug Class, 2023 to 2033
Figure 102: Middle East and Africa Market Value (US$ Million) by Application, 2023 to 2033
Figure 103: Middle East and Africa Market Value (US$ Million) by Distribution Channel , 2023 to 2033
Figure 104: Middle East and Africa Market Value (US$ Million) by Country, 2023 to 2033
Figure 105: Middle East and Africa Market Value (US$ Million) Analysis by Country, 2018 to 2033
Figure 106: Middle East and Africa Market Value Share (%) and BPS Analysis by Country, 2023 to 2033
Figure 107: Middle East and Africa Market Y-o-Y Growth (%) Projections by Country, 2023 to 2033
Figure 108: Middle East and Africa Market Value (US$ Million) Analysis by Drug Class, 2018 to 2033
Figure 109: Middle East and Africa Market Value Share (%) and BPS Analysis by Drug Class, 2023 to 2033
Figure 110: Middle East and Africa Market Y-o-Y Growth (%) Projections by Drug Class, 2023 to 2033
Figure 111: Middle East and Africa Market Value (US$ Million) Analysis by Application, 2018 to 2033
Figure 112: Middle East and Africa Market Value Share (%) and BPS Analysis by Application, 2023 to 2033
Figure 113: Middle East and Africa Market Y-o-Y Growth (%) Projections by Application, 2023 to 2033
Figure 114: Middle East and Africa Market Value (US$ Million) Analysis by Distribution Channel , 2018 to 2033
Figure 115: Middle East and Africa Market Value Share (%) and BPS Analysis by Distribution Channel , 2023 to 2033
Figure 116: Middle East and Africa Market Y-o-Y Growth (%) Projections by Distribution Channel , 2023 to 2033
Figure 117: Middle East and Africa Market Attractiveness by Drug Class, 2023 to 2033
Figure 118: Middle East and Africa Market Attractiveness by Application, 2023 to 2033
Figure 119: Middle East and Africa Market Attractiveness by Distribution Channel , 2023 to 2033
Figure 120: Middle East and Africa Market Attractiveness by Country, 2023 to 2033






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Tax Management Market Size and Share Forecast Outlook 2025 to 2035
Key Management as a Service Market
Cash Management Supplies Packaging Market Size and Share Forecast Outlook 2025 to 2035
Fuel Management Software Market Size and Share Forecast Outlook 2025 to 2035
Risk Management Market Size and Share Forecast Outlook 2025 to 2035
SBOM Management and Software Supply Chain Compliance Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Case Management Software (CMS) Market Size and Share Forecast Outlook 2025 to 2035
Farm Management Software Market Size and Share Forecast Outlook 2025 to 2035
Lead Management Market Size and Share Forecast Outlook 2025 to 2035
Pain Management Devices Market Growth - Trends & Forecast 2025 to 2035
Data Management Platforms Market Analysis and Forecast 2025 to 2035, By Type, End User, and Region
Cash Management Services Market – Trends & Forecast 2025 to 2035
CAPA Management (Corrective Action / Preventive Action) Market
Exam Management Software Market
Light Management System Market Size and Share Forecast Outlook 2025 to 2035
Labor Management System In Retail Market Size and Share Forecast Outlook 2025 to 2035
Waste Management Carbon Credit Market Size and Share Forecast Outlook 2025 to 2035
Waste Management Market Size and Share Forecast Outlook 2025 to 2035
Stool Management System Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Power Management System Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA