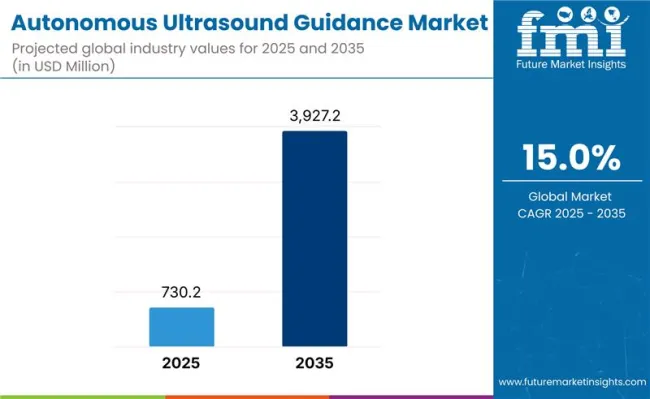
The global autonomous ultrasound guidance market is projected to grow from USD 730.2 million in 2025 to approximately USD 3,927.2 million by 2035, recording an absolute increase of USD 3,197.0 million over the forecast period. This translates into a total growth of 437.8%, with the market forecast to expand at a compound annual growth rate (CAGR) of 18.3% between 2025 and 2035.
Autonomous Ultrasound Guidance Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 730.2 million |
| Forecast Value in (2035F) | USD 3,927.2 million |
| Forecast CAGR (2025 to 2035) | 18.3% |
The overall market size is expected to grow by nearly 5.4X during the same period, supported by increasing demand for automated diagnostic solutions, growing adoption of AI-powered medical devices, and rising utilization of autonomous ultrasound systems in critical medical applications across global healthcare and diagnostic infrastructure industries.
Between 2025 and 2030, the autonomous ultrasound guidance market is projected to expand from USD 730.2 million to USD 1,693.4 million, resulting in a value increase of USD 963.2 million, which represents 30.1% of the total forecast growth for the decade. This phase of development will be shaped by increasing healthcare automation deployment, rising adoption of AI-powered diagnostic equipment in medical processes, and growing utilization in emergency care infrastructure and advanced imaging applications.
Medical device manufacturers and ultrasound technology providers are expanding their production capabilities to address the growing preference for autonomous diagnostic solutions in critical medical operations and precision imaging applications.
From 2030 to 2035, the market is forecast to grow from USD 1,693.4 million to USD 3,927.2 million, adding another USD 2,233.8 million, which constitutes 69.9% of the overall ten-year expansion. This period is expected to be characterized by the expansion of advanced autonomous ultrasound technologies, the integration of smart imaging systems with AI capabilities, and the development of specialized ultrasound solutions for emerging medical applications.
The growing emphasis on point-of-care diagnostics and autonomous medical device implementation will drive demand for intelligent ultrasound systems with advanced imaging features and enhanced diagnostic reliability.
Between 2020 and 2024, the autonomous ultrasound guidance market experienced robust growth, driven by increasing medical automation adoption and growing recognition of autonomous ultrasound's importance in critical diagnostic operations. The market developed as healthcare providers recognized the potential for AI-powered ultrasound systems to enhance diagnostic accuracy while meeting stringent medical requirements.
Technological advancement in artificial intelligence and autonomous imaging began emphasizing the critical importance of maintaining diagnostic precision while extending service capabilities and improving patient care outcomes.
Market expansion is being supported by the increasing global demand for automated diagnostic solutions and the corresponding shift toward advanced autonomous ultrasound technologies that can provide superior imaging performance while meeting stringent specifications for critical medical applications.
Modern healthcare facilities and medical device manufacturers are increasingly focused on incorporating autonomous ultrasound systems to enhance diagnostic reliability while satisfying demands for extended service capabilities and proven performance in demanding healthcare environments.
Autonomous ultrasound guidance's proven ability to deliver superior imaging accuracy, operational efficiency, and diagnostic precision makes it essential components for advanced medical operations and critical healthcare applications.
The growing emphasis on healthcare automation and smart diagnostic systems is driving demand for high-quality autonomous ultrasound solutions that can support distinctive performance characteristics and mission-critical applications across imaging, emergency care, and interventional procedure categories.
Healthcare provider preference for technologies that combine diagnostic excellence with proven reliability credentials is creating opportunities for innovative autonomous ultrasound implementations in both traditional and emerging medical applications. The rising influence of point-of-care diagnostic technologies and operational optimization is also contributing to increased adoption of intelligent ultrasound systems that can provide real-time imaging monitoring and diagnostic insights.
The market is segmented by product type, application, end user, and region. By product type, the market is divided into robotic ultrasound guidance systems, AI-enabled handheld autonomous ultrasound devices, and autonomous ultrasound image analysis software. Based on application, the market is categorized into diagnostic imaging, interventional procedures, vascular access, and fluid sampling/drainage.
By end user, the market is divided into hospitals & clinics, diagnostic imaging centers, ambulatory surgical centers, emergency & field providers, and academic & research labs. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and the Middle East & Africa.
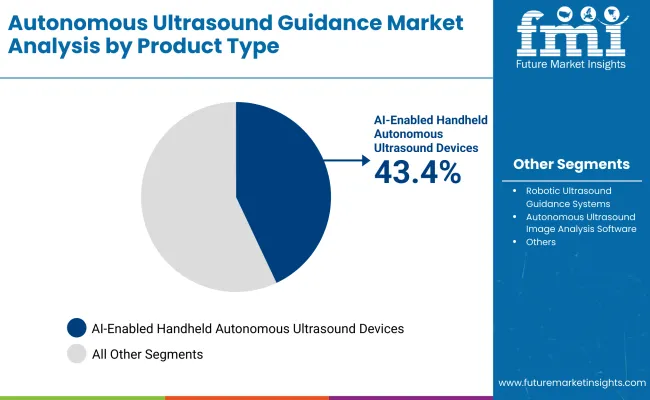
The AI-enabled handheld autonomous ultrasound devices segment is projected to account for 43.4% of the autonomous ultrasound guidance market in 2025, reaffirming its position as the leading product type category.
Healthcare providers and medical device manufacturers increasingly utilize AI-enabled handheld devices for their operational versatility, superior portability characteristics, and ease of integration in complex medical systems across diverse healthcare applications. This product type's proven design directly addresses medical requirements for reliable performance and efficient operation in point-of-care diagnostic procedures.
This segment forms the foundation of modern autonomous ultrasound applications, as it represents the configuration with the greatest adaptability and established compatibility across multiple healthcare systems. Manufacturer investments in AI algorithm optimization and handheld device engineering continue to strengthen adoption among healthcare providers.
With medical institutions prioritizing diagnostic reliability and consistent performance characteristics, AI-enabled handheld devices align with both efficiency objectives and portability requirements, making them the central component of comprehensive autonomous ultrasound strategies.
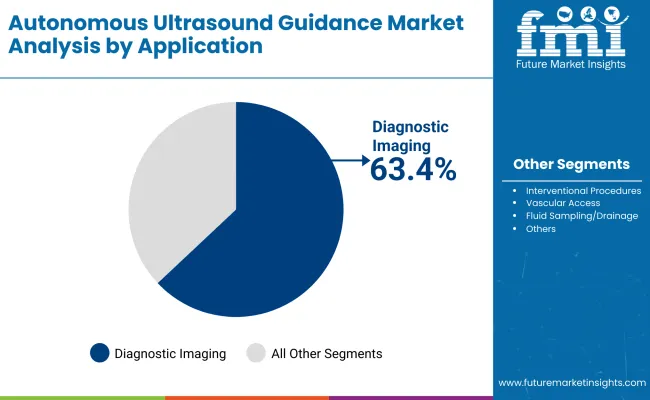
Diagnostic imaging applications represent the dominant segment of autonomous ultrasound guidance demand in 2025 with 63.4%market share, underscoring their critical role as essential applications for medical diagnostic operations.
Healthcare organizations prefer autonomous ultrasound systems for their imaging reliability, diagnostic accuracy, and ability to support both standard operations and specialized applications while offering proven effectiveness in demanding medical environments. Positioned as essential applications for autonomous ultrasound solutions, diagnostic imaging offers both operational capability and competitive advantages.
The segment is supported by continuous growth in medical imaging programs and the growing availability of specialized autonomous ultrasound variants that enable diagnostic differentiation and imaging effectiveness at the institutional level.
Additionally, healthcare providers are investing in advanced autonomous ultrasound technologies to support precision diagnostics positioning and operational accessibility. As medical automation continues to gain priority and providers seek reliable imaging technologies, diagnostic imaging applications will continue to dominate the market landscape while supporting operational excellence and diagnostic reliability strategies.
The autonomous ultrasound guidance market is advancing robustly due to increasing healthcare preference for AI-enabled diagnostic solutions and growing demand for autonomous systems that emphasize reliable operation across medical and imaging applications.
However, the market faces challenges, including integration complexity with existing medical systems, regulatory approval requirements for autonomous devices, and high initial investment costs. Innovation in smart ultrasound systems and AI-powered diagnostic integration continues to influence market development and expansion patterns.
Expansion of Point-of-Care Diagnostic Applications
The growing adoption of autonomous ultrasound guidance in point-of-care medical systems and portable diagnostic applications is enabling healthcare providers to develop capabilities that provide distinctive operational advantages while commanding premium positioning and enhanced diagnostic characteristics.
Point-of-care applications provide superior operational differentiation while allowing more sophisticated healthcare development across various medical categories and diagnostic segments. Healthcare providers are increasingly recognizing the competitive advantages of autonomous ultrasound integration for advanced diagnostic development and healthcare automation penetration.
Integration of AI-Powered Imaging Technologies
Modern autonomous ultrasound providers are incorporating AI algorithms, real-time image analysis systems, and automated diagnostic capabilities to enhance imaging performance, improve operational insights, and meet medical demands for intelligent ultrasound solutions.
These technologies enhance diagnostic efficiency while enabling new applications, including automated screening and smart diagnostic systems for healthcare delivery. Advanced technology integration also allows providers to support premium market positioning and medical trust building beyond traditional ultrasound service relationships.
| Country | CAGR (2025 to 2035) |
|---|---|
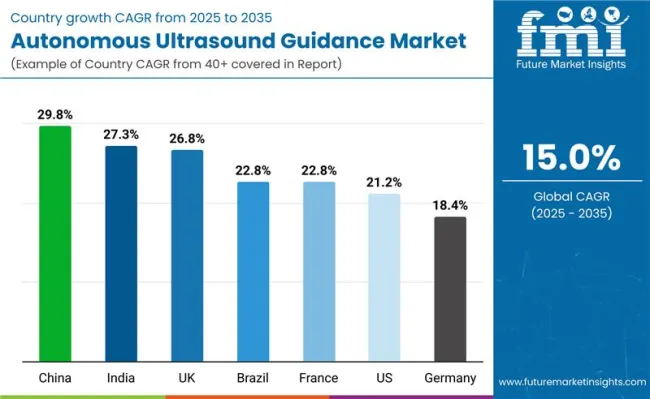
| China | 29.8% |
| India | 27.3% |
| UK | 26.8% |
| Brazil | 22.8% |
| France | 22.8% |
| USA | 21.2% |
| Germany | 18.4% |
The autonomous ultrasound guidance market is experiencing robust growth globally, with China leading at a 29.8% CAGR through 2035, driven by massive healthcare infrastructure development, rapid adoption of AI-powered medical devices, and increasing focus on advanced diagnostic solutions in expanding healthcare sectors. India follows at 27.3%, supported by digital healthcare initiatives, rising medical automation investments, and increasing demand for portable diagnostic solutions in emerging medical markets.
The UK shows growth at 26.8%, emphasizing healthcare technology excellence and autonomous diagnostic system development. Brazil and France both demonstrate 22.8% growth, prioritizing healthcare modernization and AI-powered medical device adoption.
The USA exhibits 21.2% growth, supported by advanced healthcare capabilities and established medical technology infrastructure. Germany shows 18.4% growth, focusing on precision medical technology and autonomous ultrasound manufacturing.
The report covers an in-depth analysis of 40+ countries; eight top-performing countries are highlighted below.
Revenue from autonomous ultrasound guidance in China is projected to exhibit exceptional growth with a CAGR of 29.8% through 2035, driven by the rapidly expanding healthcare infrastructure and increasing AI adoption across medical facilities seeking advanced diagnostic capabilities.
The country's growing medical technology sector and strategic healthcare digitization development are creating substantial demand for autonomous ultrasound solutions in both traditional hospital and telemedicine applications. Major medical device companies and ultrasound technology providers are establishing comprehensive production and distribution capabilities to serve both domestic healthcare requirements and export markets.
Revenue from autonomous ultrasound guidance in India is expanding at a CAGR of 27.3%, supported by digital healthcare initiatives, increasing medical technology investments, and growing focus on portable diagnostic development requiring autonomous ultrasound solutions.
The country's developing healthcare technology sector and expanding medical infrastructure are driving demand for advanced autonomous systems across both traditional and telemedicine applications. International ultrasound companies and domestic manufacturers are establishing comprehensive distribution and production capabilities to address growing market demand for intelligent diagnostic components.
Revenue from autonomous ultrasound guidance in the United Kingdom is projected to grow at a CAGR of 26.8% through 2035, driven by the country's advanced healthcare capabilities, medical technology innovation, and continued focus on autonomous diagnostic solutions for critical medical applications.
The UK's sophisticated healthcare system and willingness to invest in advanced autonomous technologies are creating substantial demand for both standard and specialized ultrasound variants. Leading healthcare organizations and technology suppliers are establishing comprehensive integration strategies to serve both medical operations and specialized diagnostic markets.
Revenue from autonomous ultrasound guidance in Brazil is projected to grow at a CAGR of 22.8% through 2035, supported by the country's healthcare modernization programs, expanding medical capabilities, and focus on diagnostic infrastructure development requiring autonomous ultrasound solutions.
Brazilian healthcare facilities prioritize operational effectiveness, diagnostic reliability, and medical capability enhancement, making autonomous ultrasound systems essential components for both domestic operations and regional healthcare applications. The country's comprehensive healthcare development programs and strategic emphasis support continued autonomous ultrasound market development.
Revenue from autonomous ultrasound guidance in France is projected to grow at a CAGR of 22.8% through 2035, supported by the country's advanced medical technology industry, healthcare equipment innovation sector, and established tradition of healthcare excellence requiring high-quality autonomous ultrasound components.
French healthcare providers and medical producers consistently demand superior autonomous systems that meet exacting quality standards for both domestic healthcare delivery and export markets. The country's position as a medical technology innovator continues to drive advancement in autonomous ultrasound applications and quality standards.
Revenue from autonomous ultrasound guidance in the United States is projected to grow at a CAGR of 21.2% through 2035, supported by the country's established healthcare infrastructure, medical device manufacturing capabilities, and focus on advanced diagnostic technologies requiring autonomous ultrasound solutions.
American healthcare organizations' focus on operational excellence and innovative medical technologies creates steady demand for reliable autonomous ultrasound systems and specialized variants. The country's attention to diagnostic quality and operational effectiveness drives consistent adoption across both traditional and specialized medical applications.
Revenue from autonomous ultrasound guidance in Germany is projected to grow at a CAGR of 18.4% through 2035, supported by the country's precision engineering industry, medical device manufacturing expertise, and established industrial tradition requiring high-quality autonomous ultrasound components.
German healthcare organizations' focus on operational excellence and proven technologies creates steady demand for reliable autonomous ultrasound systems and specialized variants. The country's attention to component quality and operational effectiveness drives consistent adoption across both traditional and specialized medical applications.
The autonomous ultrasound guidance market in Europe is projected to grow from USD 192.2 million in 2025 to USD 1,076.4 million by 2035, registering a CAGR of 18.8% over the forecast period. Germany is expected to maintain its leadership position with a 28.1% market share in 2025, declining slightly to 27.9% by 2035, supported by its advanced medical technology sector and ultrasound equipment manufacturing capabilities serving European and international markets.
The United Kingdom follows with a 18.1% share in 2025, projected to reach 17.4% by 2035, driven by established healthcare expertise and autonomous medical technology capabilities. Spain holds a 14.5% share in 2025, expected to reach 16.6% by 2035, supported by growing healthcare automation programs and medical technology development.
France commands a 12.5% share in 2025, projected to reach 12.3% by 2035, while Italy accounts for 7.5% in 2025, expected to reach 8.7% by 2035. The Nordic Countries maintain a 9.0% share in 2025, declining to 7.2% by 2035. BENELUX holds 6.9% in 2025, growing to 7.5% by 2035.
The Rest of Western Europe region is anticipated to hold 3.5% in 2025, declining to 2.4% by 2035, attributed to mixed growth patterns with steady expansion in some markets balanced by consolidation in smaller Western European countries implementing healthcare digitization programs.
In Japan, adoption centers on AI-enabled handheld autonomous ultrasound devices-the leading global product type (43.4% in 2025)-to extend imaging capacity across acute care, community clinics, and aging-care settings without sacrificing diagnostic rigor.
Hospitals pair handhelds with autonomous image-analysis software for triage, vascular access, and peri-procedural guidance, while robotics expertise supports pilots in robotic ultrasound guidance for standardized scans. With tight quality regimes and workforce constraints, providers prioritize systems that deliver reproducible image acquisition, real-time decision support, and seamless integration into PACS/EHR and emergency workflows.Adoption Insights:
In South Korea, rapid hospital digitization and a strong med-tech supply base drive uptake of autonomous ultrasound across ER, ICU, and ambulatory centers. Providers lean on AI guidance for standardized acquisition and automated measurements to reduce variability and accelerate throughput in diagnostic imaging-the dominant application-while expanding into fluid drainage and bedside procedures.
Local manufacturers and integrators emphasize cost-efficient deployment, cybersecurity, and remote support, positioning systems for regional export as volumes rise from 2025 to 2035 (market CAGR 18.3% globally):
The autonomous ultrasound guidance market is characterized by competition among established medical device manufacturers, specialized ultrasound technology providers, and integrated healthcare equipment suppliers.
Companies are investing in advanced AI technologies, autonomous imaging systems, real-time analysis capabilities, and comprehensive diagnostic platforms to deliver consistent, high-performance, and reliable autonomous ultrasound solutions. Innovation in machine learning algorithms, smart imaging systems, and specialized medical applications is central to strengthening market position and healthcare provider confidence.
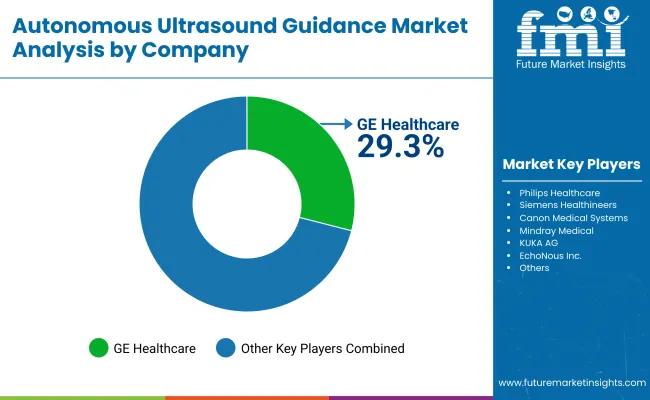
GE Healthcare leads the market with a strong focus on autonomous ultrasound innovation and medical device manufacturing, offering comprehensive ultrasound solutions that emphasize diagnostic accuracy and operational reliability. Philips provides integrated imaging and medical device capabilities with a focus on advanced healthcare technology and global distribution networks.
Siemens Healthineers delivers precision ultrasound solutions with a focus on medical excellence and specialized applications. Canon Medical Systems specializes in high-performance imaging solutions with emphasis on healthcare-grade quality and critical applications. Mindray Medical focuses on medical device technology and emerging market development. KUKA AG emphasizes robotic integration with a focus on autonomous medical applications and automation excellence.
The global autonomous ultrasound guidance market represents a critical technology segment within medical imaging and diagnostic automation, projected to grow from USD 730.2 million in 2025 to USD 3,927.2 million by 2035 at a CAGR of 18.3%. AI-enabled handheld devices dominate with 43.4% market share, serving primarily diagnostic imaging applications that demand exceptional imaging accuracy, operational precision, and extended service capabilities.
The market's expansion is driven by healthcare automation adoption, advanced medical imaging requirements, and growing emphasis on point-of-care diagnostics across critical healthcare applications. Achieving sustained growth requires coordinated efforts across healthcare policy makers, regulatory organizations, medical device manufacturers, healthcare integrators, and investment providers.
Healthcare Infrastructure Investment: Establish dedicated medical technology zones and innovation hubs with specialized facilities for autonomous ultrasound development, including advanced imaging centers, diagnostic testing laboratories, and skilled workforce training institutes to support domestic medical device manufacturing capabilities.
R&D and Innovation Support: Fund research programs focused on advanced autonomous ultrasound algorithms, smart imaging technologies with AI integration, and automated diagnostic systems. Support collaborative projects between universities, research institutes, and medical device companies to develop next-generation autonomous ultrasound solutions.
Skills Development Programs: Invest in technical education and vocational training programs that develop expertise in medical imaging technology, ultrasound physics, AI algorithm development, and advanced diagnostic techniques required for autonomous ultrasound development and implementation.
Export Promotion & Trade Facilitation: Create export incentive schemes for autonomous ultrasound manufacturers, establish trade promotion councils to support international market development, and negotiate favorable trade agreements that reduce barriers for medical device component imports and exports.
Medical Standards & Certification: Develop national quality standards aligned with international medical specifications (FDA, CE, ISO) and establish accredited testing facilities that enable domestic autonomous ultrasound manufacturers to compete in global markets with certified quality credentials.
Technical Standards Development: Establish comprehensive quality standards for autonomous ultrasound solutions covering imaging accuracy, safety specifications, performance metrics, and interoperability criteria that ensure consistent quality across manufacturers and enable reliable healthcare procurement decisions.
Testing and Certification Protocols: Develop standardized testing methodologies for autonomous ultrasound performance evaluation including clinical validation, safety assessment, accuracy measurement procedures, and quality assurance frameworks that support healthcare adoption decisions.
Best Practices Documentation: Create technical guidelines for autonomous ultrasound selection, implementation procedures, integration protocols, and maintenance methodologies that help healthcare providers optimize system performance and extend operational effectiveness across diverse medical applications.
Professional Certification Programs: Establish technical certification courses for autonomous ultrasound specialists, medical imaging engineers, and quality control personnel to ensure industry-wide competency in autonomous ultrasound technology and application expertise.
Market Intelligence & Analytics: Provide regular industry reports covering technology trends, market dynamics, competitive analysis, and emerging applications to guide strategic planning for manufacturers, healthcare providers, and technology integrators across global markets.
Advanced Autonomous Technologies: Develop cutting-edge autonomous ultrasound capabilities including advanced AI algorithms, real-time image processing, automated diagnostic features, and intelligent analysis systems that ensure consistent delivery of high-performance medical solutions meeting stringent healthcare specifications.
Smart Imaging Integration: Incorporate IoT sensors, cloud connectivity capabilities, and predictive analytics into autonomous ultrasound systems to enable remote monitoring, automated diagnostics scheduling, and operational optimization for healthcare providers and medical institutions.
Medical Innovation: Advanced imaging algorithms, including specialized diagnostic models, automated analysis systems, predictive healthcare analytics, and clinical decision support tools that improve diagnostic accuracy, reduce medical errors, extend service capabilities, and enhance performance in demanding healthcare environments.
Modular System Platforms: Create standardized autonomous ultrasound families and modular system architectures that enable rapid customization for specific medical applications while maintaining economies of scale in development and reducing implementation costs for specialized healthcare requirements.
Technical Support Infrastructure: Establish comprehensive customer support networks, including medical application engineering services, on-site technical assistance, training programs, and digital tools that help healthcare providers select, implement, and maintain optimal autonomous ultrasound solutions.
Integrated Diagnostic Strategies: Develop autonomous ultrasound integration approaches that optimize healthcare delivery around imaging capabilities, including workflow optimization analysis, diagnostic enhancement, and operational management to maximize system performance and healthcare service reliability.
Technology Partnership Development: Build strategic relationships with autonomous ultrasound manufacturers that provide early access to new technologies, collaborative solution development opportunities, and guaranteed technology supply security for critical healthcare applications requiring specialized imaging solutions.
Predictive Healthcare Integration: Implement condition monitoring systems and predictive analytics capabilities that leverage smart autonomous ultrasound technologies to optimize patient care, reduce healthcare costs, and improve overall operational effectiveness.
Application-Specific Solutions: Develop specialized autonomous ultrasound requirements for specific medical applications including emergency diagnostics, interventional procedures, vascular access applications, and point-of-care systems that require unique performance characteristics.
Quality Assurance Programs: Establish comprehensive technology qualification procedures including performance validation, reliability testing, and technology provider auditing processes that ensure consistent quality and performance across autonomous ultrasound supply chains.
Technology Development Expansion: Finance advanced autonomous ultrasound development facilities, automated manufacturing systems, and precision technology investments that enable medical device companies to scale development capabilities and meet growing global demand for intelligent imaging components.
Medical Innovation Funding: Provide venture capital and growth funding for autonomous ultrasound startups developing smart imaging systems, advanced diagnostic algorithms, innovative healthcare platforms, and digital solutions that advance autonomous ultrasound performance and capabilities.
Market Expansion Capital: Support international market development initiatives, including regional technology facilities, distribution networks, technical support infrastructure, and local partnerships in high-growth markets like Asia-Pacific and emerging economies.
Healthcare Integration Support: Finance vertical integration strategies that combine autonomous ultrasound development with healthcare delivery, medical technology manufacturing, and service capabilities to create more efficient and competitive medical device supply chains.
Industry Consolidation Support: Facilitate strategic mergers and acquisitions that create larger, more capable autonomous ultrasound organizations with enhanced R&D capabilities, broader technology portfolios, and global market reach necessary to serve major healthcare providers and medical institutions effectively.
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 730.2 Million |
| Product Type | Robotic Ultrasound Guidance Systems, AI-Enabled Handheld Autonomous Ultrasound Devices, Autonomous Ultrasound Image Analysis Software |
| Application | Diagnostic Imaging, Interventional Procedures, Vascular Access, Fluid Sampling/Drainage |
| End User | Hospitals & Clinics, Diagnostic Imaging Centers , Ambulatory Surgical Centers , Emergency & Field Providers, Academic & Research Labs |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, China, India, Germany, France, UK, Spain, Brazil, Japan, South Korea and 40+ countries |
| Key Companies Profiled | GE Healthcare, Siemens Healthineers , Canon Medical Systems, Mindray Medical, KUKA AG, EchoNous Inc., and other specialized ultrasound technology providers |
| Additional Attributes | Dollar sales by product type and application, regional demand trends, competitive landscape, technological advancements in autonomous ultrasound, smart imaging system integration, predictive diagnostic programs, and healthcare automation strategies |
North America
Europe
East Asia
South Asia & Pacific
Latin America
Middle East & Africa
The global Autonomous Ultrasound Guidance market is valued at USD 730.2 million in 2025.
The size of the Autonomous Ultrasound Guidance market is projected to reach USD 3,927.2 million by 2035.
The Autonomous Ultrasound Guidance market is expected to grow at a 15.0% CAGR between 2025 and 2035.
The key product type segments in the Autonomous Ultrasound Guidance market are Robotic Ultrasound Guidance Systems, AI-Enabled Handheld Autonomous Ultrasound Devices, and Autonomous Ultrasound Image Analysis Software.
In terms of product type, the AI-Enabled Handheld Autonomous Ultrasound Devices segment is set to command the dominant share in the Autonomous Ultrasound Guidance market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Autonomous Driving Simulation Tester Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Aerial Robot Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Driving Virtual Simulation Platform Market Forecast and Outlook 2025 to 2035
Autonomous Imaging Market Size and Share Forecast Outlook 2025 to 2035
Autonomous AI Powered Ophthalmology Screening Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Radiology Systems Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Agents Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Parking Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Trucks Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Drone Platform Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Mobile Robots for Logistics and Warehousing Market Size and Share Forecast Outlook 2025 to 2035
Autonomous Vehicles Market Growth - Trends & Forecast 2025 to 2035
Autonomous Crane Market Growth - Trends & Forecast 2025 to 2035
Autonomous Forklift Market Growth – Trends & Forecast 2024-2034
Autonomous Robot Toys Market
Autonomous Intelligent Vehicle Market
USA Autonomous Crane Market Analysis – Growth, Trends & Forecast 2025-2035
China Autonomous Crane Market Size and Share Forecast Outlook 2025 to 2035
India Autonomous Crane Market Growth – Innovations, Trends & Forecast 2025-2035
Japan Autonomous Crane Market Report – Growth, Trends & Forecast 2025-2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA