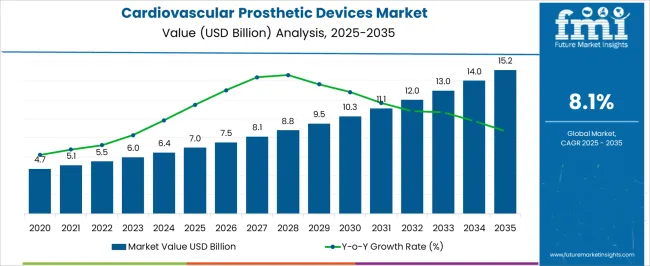
The Cardiovascular Prosthetic Devices Market is estimated to be valued at USD 7.0 billion in 2025 and is projected to reach USD 15.2 billion by 2035, registering a compound annual growth rate (CAGR) of 8.1% over the forecast period.
| Metric | Value |
|---|---|
| Cardiovascular Prosthetic Devices Market Estimated Value in (2025 E) | USD 7.0 billion |
| Cardiovascular Prosthetic Devices Market Forecast Value in (2035 F) | USD 15.2 billion |
| Forecast CAGR (2025 to 2035) | 8.1% |
The cardiovascular prosthetic devices market is experiencing significant growth, driven by rising prevalence of cardiovascular diseases and increased demand for minimally invasive treatment options. Growing awareness about heart health and early intervention is encouraging adoption of prosthetic valves, stents, and other cardiovascular implants across developed and emerging markets. Advancements in biomedical engineering, material science, and device miniaturization are enhancing product efficacy, durability, and patient outcomes.
Investments in healthcare infrastructure and technological innovation are expanding access to these devices, particularly in hospitals and specialized cardiac centers. The ongoing development of next-generation prosthetic solutions with improved biocompatibility and lower risk of thrombosis is further driving adoption. Aging populations, increasing surgical interventions, and favorable reimbursement policies are contributing to market expansion.
As cardiovascular diseases remain a leading cause of mortality worldwide, prosthetic devices are becoming critical tools for treatment and management, positioning the market for sustained growth over the coming decade Rising focus on patient-centric care, safety, and long-term clinical outcomes continues to influence product development and uptake.
The cardiovascular prosthetic devices market is segmented by product, application, end user, and geographic regions. By product, cardiovascular prosthetic devices market is divided into Valves and Pacemakers. In terms of application, cardiovascular prosthetic devices market is classified into Surgery and Research. Based on end user, cardiovascular prosthetic devices market is segmented into Hospitals, Cardiac Centers, Ambulatory Surgical Centers, and Specialty Clinics. Regionally, the cardiovascular prosthetic devices industry is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
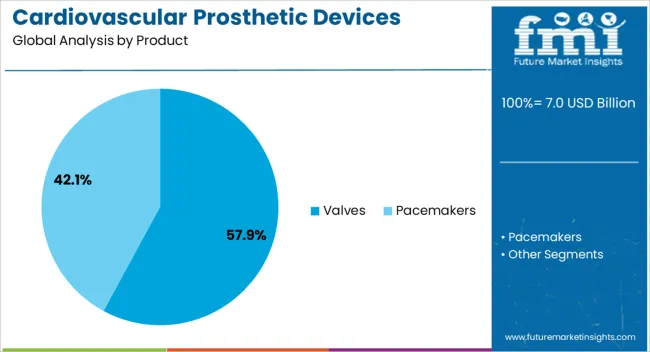
The valves product segment is projected to hold 57.9% of the cardiovascular prosthetic devices market revenue share in 2025, establishing it as the leading product type. This dominance is being driven by the high prevalence of valvular heart diseases and the increasing demand for durable, reliable prosthetic valve solutions that can improve patient survival and quality of life. Technological advancements in material composition, including biocompatible metals and polymers, are enhancing device longevity and reducing complications.
Both surgical and transcatheter valve replacements are being widely adopted, with flexible procedural approaches enabling access to high-risk patients. The segment benefits from strong clinical validation and a broad base of healthcare professionals trained in valve implantation techniques.
Hospitals and specialized cardiac centers are prioritizing the use of prosthetic valves due to their demonstrated efficacy in restoring normal cardiac function and reducing long-term morbidity Rising investment in research and development is expected to further improve valve design, performance, and patient outcomes, maintaining the segment’s leading position in the market.
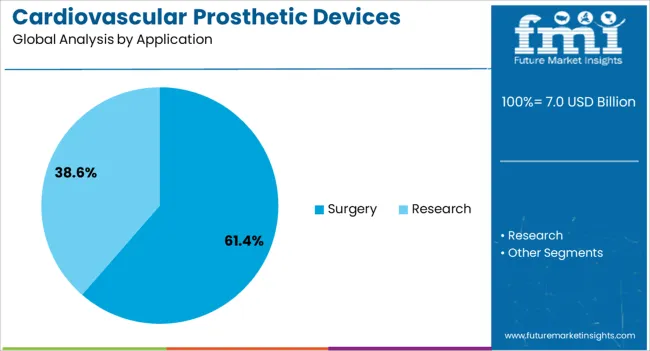
The surgery application segment is expected to account for 61.4% of the cardiovascular prosthetic devices market revenue share in 2025, positioning it as the leading application. Its dominance is being influenced by the growing number of cardiac surgical procedures, including valve replacement, bypass surgery, and minimally invasive interventions. Surgical adoption is being reinforced by improvements in procedural precision, device design, and post-operative patient care, which enhance outcomes and reduce recovery time.
Surgeons increasingly rely on advanced prosthetic devices that provide superior hemodynamic performance and durability, supporting patient-specific treatment strategies. Hospitals and cardiac centers are investing in state-of-the-art operating theaters and training programs to ensure successful implementation.
The segment benefits from the integration of imaging technologies and robotic-assisted surgical techniques, which improve implantation accuracy and safety Rising prevalence of heart conditions, aging populations, and demand for long-term functional restoration are expected to sustain growth in surgical applications of cardiovascular prosthetic devices.
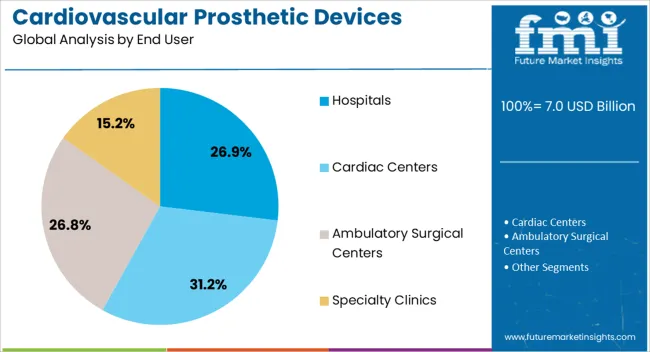
The hospitals end-user segment is projected to hold 26.9% of the cardiovascular prosthetic devices market revenue share in 2025, making it the leading end-use category. Hospitals are being prioritized as the primary adoption channel due to their advanced infrastructure, availability of skilled cardiologists and cardiac surgeons, and capacity to perform complex procedures. Large-scale hospitals and specialized cardiac centers are increasingly integrating high-quality prosthetic devices to improve clinical outcomes and operational efficiency.
Hospitals benefit from comprehensive procurement systems and established supply chains, which ensure timely availability of prosthetic valves and other cardiovascular implants. The segment’s growth is also being reinforced by investments in hospital expansion, modernization, and adoption of minimally invasive surgical technologies.
Rising patient volumes, increasing surgical interventions, and emphasis on high-quality care standards are driving sustained adoption As healthcare facilities continue to upgrade cardiac care capabilities and expand treatment offerings, the hospitals segment is expected to remain the largest end-user contributor to market revenue.
The market for cardiovascular prosthetic devices is driven owing to the increasing prevalence of atrial septal defect (ASD) and valvular disease, the increasing rates of cardiovascular diseases, and the geriatric population. The new technology innovations and growing adaptation rates of these devices are likely to propel the market in the forecast period.
Additionally, constant growth in the elderly population, increasing preference for minimally-invasive procedures, technological upgradation resulting in the development of effective devices, and an upsurge in obesity followed by continuous product development are expected to boost the market for cardiovascular prosthetic devices. Moreover, the rising prevalence of hypertension among people is predicted to rise in the cardiovascular prosthetic market in the projected period.
Due to the high prevalence of cardiovascular disorders such as rheumatic diseases and valvular disorders and the growing demand for the devices in the North American countries are expected to develop the highest market for cardiovascular prosthetic devices in the projected years.
Europe is also expected to grow with a lucrative market share. With the rising prevalence of heart valve disease and irregular heart rhythm disease, along with the quick adoption of improved prosthetic devices, the market is expected to grow in the near future.
The rising population and developing healthcare infrastructure in the developing countries in the Asian region are predicted to expand the growth of the cardiovascular prosthetic devices market. Adding to that the developing reimbursement policies in these is propelling the market growth.
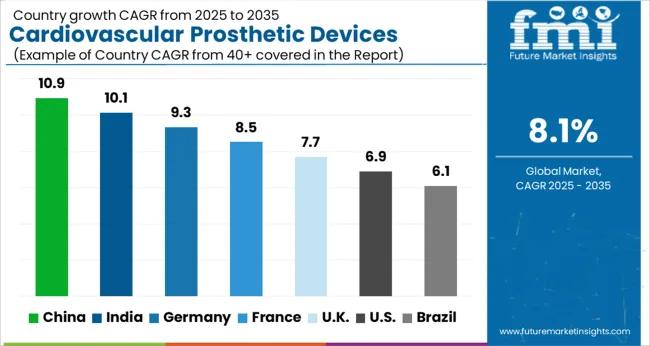
| Country | CAGR |
|---|---|
| China | 10.9% |
| India | 10.1% |
| Germany | 9.3% |
| France | 8.5% |
| UK | 7.7% |
| USA | 6.9% |
| Brazil | 6.1% |
The Cardiovascular Prosthetic Devices Market is expected to register a CAGR of 8.1% during the forecast period, exhibiting varied country level momentum. China leads with the highest CAGR of 10.9%, followed by India at 10.1%. Developed markets such as Germany, France, and the UK continue to expand steadily, while the USA is likely to grow at consistent rates. Brazil posts the lowest CAGR at 6.1%, yet still underscores a broadly positive trajectory for the global Cardiovascular Prosthetic Devices Market. In 2024, Germany held a dominant revenue in the Western Europe market and is expected to grow with a CAGR of 9.3%. The USA Cardiovascular Prosthetic Devices Market is estimated to be valued at USD 2.6 billion in 2025 and is anticipated to reach a valuation of USD 5.0 billion by 2035. Sales are projected to rise at a CAGR of 6.9% over the forecast period between 2025 and 2035. While Japan and South Korea markets are estimated to be valued at USD 344.3 million and USD 216.0 million respectively in 2025.
| Item | Value |
|---|---|
| Quantitative Units | USD 7.0 Billion |
| Product | Valves and Pacemakers |
| Application | Surgery and Research |
| End User | Hospitals, Cardiac Centers, Ambulatory Surgical Centers, and Specialty Clinics |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Canada, Germany, France, United Kingdom, China, Japan, India, Brazil, South Africa |
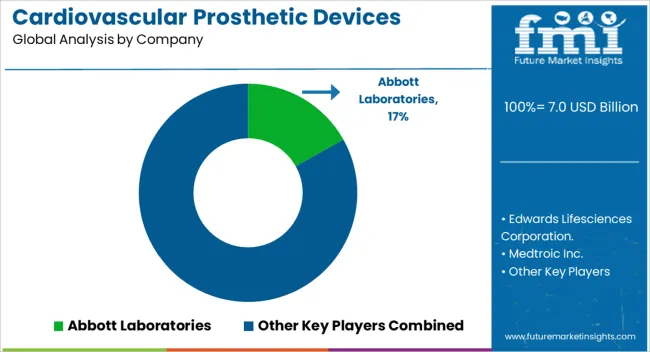
| Key Companies Profiled | Abbott Laboratories, Edwards Lifesciences Corporation., Medtroic Inc., W. L. Gore & Associates, Inc., Lifetech Scientific Co., Ltd., Abiomed, Inc., BIOTRONIK SE & Co. KG, Pacetronix, Meril Life Sciences Pvt. Ltd., and Venus Medtech |
The global cardiovascular prosthetic devices market is estimated to be valued at USD 7.0 billion in 2025.
The market size for the cardiovascular prosthetic devices market is projected to reach USD 15.2 billion by 2035.
The cardiovascular prosthetic devices market is expected to grow at a 8.1% CAGR between 2025 and 2035.
The key product types in cardiovascular prosthetic devices market are valves and pacemakers.
In terms of application, surgery segment to command 61.4% share in the cardiovascular prosthetic devices market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Cardiovascular Devices Market Size and Share Forecast Outlook 2025 to 2035
Cardiovascular Surgical Devices Market Size and Share Forecast Outlook 2025 to 2035
Cardiovascular Repair & Reconstruction Devices Market – Growth & Trends 2025 to 2035
Demand for Cardiovascular Repair & Reconstruction Devices in Japan Size and Share Forecast Outlook 2025 to 2035
Cardiovascular CT Systems Market Size and Share Forecast Outlook 2025 to 2035
Prosthetics and Orthotics Market - Growth & Future Trends 2025 to 2035
Cardiovascular Ultrasound Market - Demand & Innovations 2025 to 2035
Cardiovascular Enterprise Viewer Market Insights – Growth & Forecast 2025 to 2035
Cardiovascular Needle Market – Growth & Forecast 2025 to 2035
Cardiovascular Diagnostics Market Report- Trends & Innovations 2025 to 2035
Prosthetic Heart Valve Market is segmented by product & end user from 2025 to 2035
Competitive Overview of Neuroprosthetics Companies
FBAR Devices Market
Snare devices Market
C-Arms Devices Market Size and Share Forecast Outlook 2025 to 2035
Timing Devices Market Analysis - Size, Growth, & Forecast Outlook 2025 to 2035
Spinal Devices Market Size and Share Forecast Outlook 2025 to 2035
Hearing Devices 3D Printing Market Size and Share Forecast Outlook 2025 to 2035
Medical Devices Market Size and Share Forecast Outlook 2025 to 2035
Network Devices Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA