The chloramphenicol test kits market is forecasted to grow from USD 127.8 million in 2025 to USD 291.7 million by 2035, reflecting a CAGR of 8.6%. Demonstrating the accelerating adoption of advanced analytical systems and detection technologies across laboratories, food processing facilities, and pharmaceutical manufacturers worldwide.
The first half of the decade (2025-2030) will witness the market climbing from USD 127.8 million to approximately USD 192.5 million, adding USD 64.7 million in value, which constitutes 39.5% of the total forecast growth period. This phase will be characterized by the rapid adoption of automated testing systems, driven by increasing food safety regulations and analytical testing modernization programs worldwide. Advanced detection capabilities and integrated workflow features will become standard expectations rather than premium options.
The latter half (2030-2035) will witness steady growth from USD 192.5 million to USD 291.7 million, representing an addition of USD 99.2 million or 60.5% of the decade's expansion. This period will be defined by mass market penetration of comprehensive analytical diagnostic platforms, integration with laboratory information management systems, and seamless compatibility with existing testing infrastructure. The market trajectory signals fundamental shifts in how laboratories approach antibiotic residue detection and testing, with participants positioned to benefit from constant demand across multiple application segments.
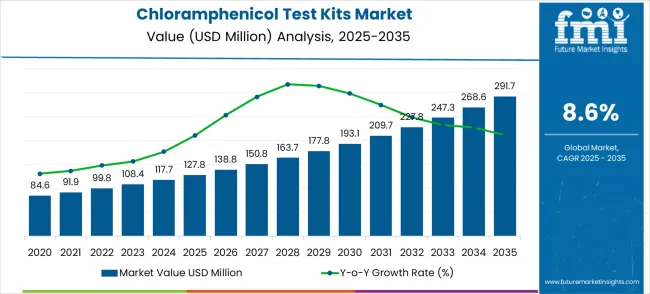
The market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its technology adoption phase, expanding from USD 127.8 million to USD 192.5 million with steady annual increments averaging 8.4% growth. This period showcases the transition from basic analytical support systems to advanced testing platforms with enhanced sensitivity capabilities and integrated laboratory workflow systems becoming mainstream features.
The 2025-2030 phase adds USD 64.7 million to market value, representing 39.5% of total decade expansion. Market maturation factors include standardization of analytical testing protocols, declining automation costs for laboratory systems, and increasing food safety professional awareness of test kit benefits reaching 95-98% accuracy in detection applications. Competitive landscape evolution during this period features established biotechnology companies like Creative Diagnostics and PerkinElmer expanding their analytical testing portfolios while new entrants focus on specialized detection algorithms and enhanced integration capabilities.
From 2030 to 2035, market dynamics shift toward advanced integration and multi-platform deployment, with growth accelerating from USD 192.5 million to USD 291.7 million, adding USD 99.2 million or 60.5% of total expansion. This phase transition logic centers on comprehensive analytical platforms, integration with laboratory information systems, and deployment across diverse testing specialties, becoming standard rather than specialized applications. The competitive environment matures with focus shifting from basic detection capability to comprehensive analytical ecosystems and integration with automated testing and quality management platforms.
| Metric | Value |
|---|---|
| $ Market Value (2025) → | USD 127.8 million |
| $ Market Forecast (2035) ↑ | USD 291.7 million |
| # Growth Rate ★ | 8.6% CAGR |
| Leading Product Type → | ELISA Kits |
| Primary Application → | Food Safety |
The market demonstrates strong fundamentals, with the ELISA Kits product type capturing a dominant share due to its advanced sensitivity features and cost-effective implementation capabilities. Food Safety applications drive primary demand, supported by increasing regulatory compliance spending on analytical testing tools and residue detection enhancement systems. Geographic expansion remains concentrated in developed markets with established laboratory infrastructure, while emerging economies show accelerating adoption rates driven by food safety modernization and rising analytical testing budgets.
Market expansion rests on three fundamental shifts driving adoption across food safety and analytical sectors. 1. Detection accuracy demand creates compelling operational advantages through analytical test systems that provide immediate diagnostic assistance without human error risks, enabling laboratory professionals to identify contamination earlier while maintaining analytical superiority and reducing false negative risks. 2. Food safety regulation programs accelerate as testing facilities worldwide seek advanced analytical systems that complement traditional testing methods, enabling precise residue detection and monitoring applications that align with regulatory standards and compliance requirements. 3. Laboratory infrastructure enhancement drives adoption from food processors and analytical centers requiring effective detection tools that minimize interpretation errors while maintaining testing quality during complex analytical procedures and quality assurance planning.
The growth faces headwinds from regulatory compliance challenges that vary across jurisdictions regarding the deployment of analytical testing systems and data validation protocols, which may limit operational flexibility in certain laboratory environments. Technical limitations also persist regarding detection sensitivity and integration complexity that may reduce system performance with legacy laboratory equipment or non-standardized sample formats that limit analytical processing capabilities.
Primary Classification: The market segments by product type into ELISA Kits, Colloidal Gold Test Kits, and Other categories, representing the evolution from traditional detection methods to advanced analytical solutions for comprehensive testing coverage.
Secondary Breakdown: Application segmentation divides the market into Food Safety, Drug Regulation, and Scientific Research sectors, reflecting distinct requirements for contamination detection, pharmaceutical quality control, regulatory compliance, and specialized research applications.
Regional Classification: Geographic distribution covers North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by food safety modernization programs.
The segmentation structure reveals technology progression from traditional manual testing toward integrated automated platforms with enhanced sensitivity and accessibility capabilities, while application diversity spans from food safety testing to comprehensive pharmaceutical and research solutions requiring precise analytical assistance.
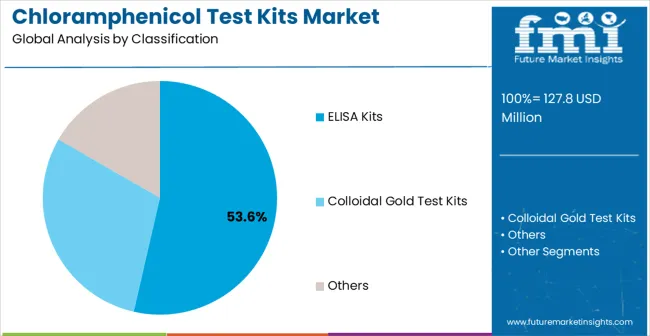
Market Position: ELISA Kits command the leading position in the market with approximately 53.6% market share through advanced sensitivity features, including quantitative detection, validated protocols, and automated processing capabilities that enable testing facilities to deploy analytical solutions across diverse laboratory environments without significant methodology changes.
Value Drivers: The segment benefits from laboratory preference for validated testing systems that provide reliable quantitative results without requiring extensive method development or validation support. ELISA design features enable deployment in food testing labs, pharmaceutical facilities, and research institutions where accuracy and regulatory compliance represent critical operational requirements.
Competitive Advantages: ELISA systems differentiate through established validation, regulatory acceptance, and integration with existing laboratory workflows that enhance analytical effectiveness while maintaining cost-effective operational profiles suitable for testing facilities of all sizes.
Key market characteristics:
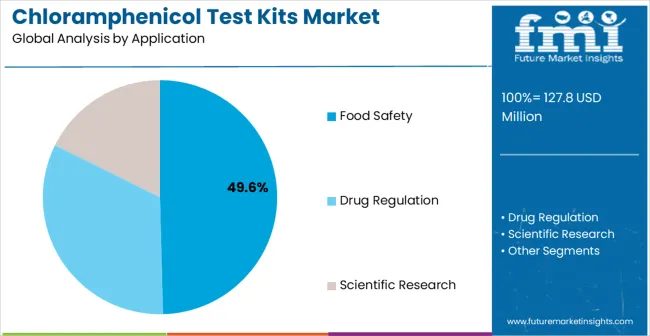
Market Context: Food Safety applications dominate the market with approximately 49.6% market share due to widespread adoption of residue testing systems and increasing focus on contamination detection, regulatory compliance, and quality assurance applications that minimize food safety risks while maintaining analytical effectiveness.
Appeal Factors: Food safety customers prioritize system accuracy, regulatory compliance, and integration with existing quality management infrastructure that enables coordinated testing across multiple food categories. The segment benefits from substantial regulatory budgets and modernization programs that prioritize analytical tool acquisition for improved consumer protection and operational efficiency.
Growth Drivers: Food safety regulation programs incorporate test kits as standard equipment for residue detection and quality assurance applications. At the same time, increasing food safety awareness is driving demand for analytical capabilities that comply with regulatory requirements and minimize contamination risks.
Market Challenges: Regulatory compliance requirements and method validation processes may limit system deployment in certain testing environments or analytical scenarios.
Application dynamics include:
Growth Accelerators: Food safety regulations drive primary adoption as test kit systems provide analytical assistance capabilities that enable accurate residue detection without human error risks, supporting quality decision-making and compliance missions that require precise contamination assessment. The demand for laboratory infrastructure accelerates market expansion as testing providers seek effective analytical enhancement tools that minimize detection errors while maintaining testing effectiveness during complex quality procedures and compliance scenarios. Regulatory spending increases worldwide, creating constant demand for analytical testing systems that complement traditional laboratory equipment and provide testing flexibility in complex regulatory environments.
Growth Inhibitors: Regulatory compliance challenges vary across jurisdictions regarding the deployment of analytical testing systems and data validation protocols, which may limit operational flexibility and market penetration in regions with restrictive laboratory technology regulations. Technical performance limitations persist regarding detection sensitivity and system integration that may reduce effectiveness with legacy laboratory equipment, non-standardized sample formats, or complex analytical workflows that limit processing capabilities. Market fragmentation across multiple regulatory standards and compliance requirements creates compatibility concerns between different analytical system providers and existing laboratory infrastructure.
Market Evolution Patterns: Adoption accelerates in food safety and analytical sectors where detection accuracy justifies system costs, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by food safety modernization and laboratory infrastructure development. Technology development focuses on enhanced detection sensitivity, improved integration capabilities, and compatibility with diverse analytical systems that optimize laboratory workflow and testing effectiveness. The market could face disruption if alternative detection technologies or regulatory restrictions significantly limit the deployment of test kits in analytical or compliance applications.
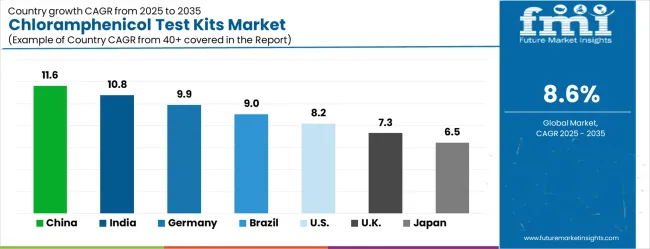
The market demonstrates varied regional dynamics with Growth Leaders including China (11.6% CAGR) and India (10.8% CAGR) driving expansion through food safety modernization and laboratory infrastructure development. Steady Performers encompass Germany (9.9% CAGR), Brazil (9.0% CAGR), and the USA (8.2% CAGR), benefiting from established analytical industries and advanced testing technology adoption. Emerging Markets feature the UK (7.3% CAGR) and Japan (6.5% CAGR), where specialized analytical applications and laboratory technology integration support consistent growth patterns.
| Country | CAGR (2025-2035) |
|---|---|
| China | 11.6% |
| India | 10.8% |
| Germany | 9.9% |
| Brazil | 9.0% |
| USA | 8.2% |
| UK | 7.3% |
| Japan | 6.5% |
Regional synthesis reveals Asia-Pacific markets leading growth through food safety modernization and laboratory infrastructure development, while European countries maintain steady expansion supported by analytical technology advancement and EU regulatory standardization requirements. North American markets show moderate growth driven by compliance applications and analytical technology integration trends.
China establishes market leadership through aggressive food safety modernization programs and comprehensive laboratory infrastructure development, integrating advanced test kit systems as standard components in food safety testing and quality control applications. The country's 11.6% CAGR through 2035 reflects government initiatives promoting analytical testing technology and domestic laboratory capabilities that mandate advanced detection systems in food processing and testing installations. Growth concentrates in major industrial centers, including Beijing, Shanghai, and Guangzhou, where analytical technology development showcases integrated testing systems that appeal to domestic laboratories seeking advanced detection capabilities and food safety enhancement applications.
Chinese manufacturers are developing cost-effective test kit solutions that combine domestic production advantages with advanced features, including precision detection algorithms and comprehensive analytical capabilities. Distribution channels through government procurement and laboratory equipment suppliers expand market access, while government funding for food safety technology development supports adoption across diverse analytical and quality testing segments.
Strategic Market Indicators:
In New Delhi, Mumbai, and Bangalore, testing facilities and analytical laboratories are implementing advanced test kit systems as standard equipment for food safety analysis and quality control applications, driven by increasing regulatory spending and modernization programs that prioritize the use of analytical testing capabilities. The market is projected to demonstrate a 10.8% CAGR through 2035, supported by government food safety initiatives and laboratory infrastructure development programs that promote advanced analytical tools for testing facilities and quality control centers. Indian laboratories are adopting test kit systems that provide precise detection capabilities and analytical enhancement features, particularly appealing in agricultural regions where testing accuracy represents critical operational requirements.
Market expansion benefits from growing analytical technology capabilities and international technology transfer agreements that enable domestic development of advanced testing systems for food safety and quality applications. Technology adoption follows patterns established in laboratory services, where detection accuracy and regulatory compliance drive procurement decisions and operational deployment.
Market Intelligence Brief:
Germany's advanced analytical technology market demonstrates sophisticated test kit deployment with documented testing effectiveness in laboratory analytical departments and food safety centers through integration with existing quality systems and regulatory infrastructure. The country leverages engineering expertise in analytical technology and laboratory systems integration to maintain a 9.9% CAGR through 2035. Testing centers, including Munich, Berlin, and Hamburg, showcase premium installations where test kits integrate with comprehensive laboratory information systems and quality management platforms to optimize detection accuracy and analytical workflow effectiveness.
German analytical technology providers prioritize system reliability and EU regulatory compliance in test kit development, creating demand for premium systems with advanced features, including method validation and integration with European laboratory standards. The market benefits from established analytical industry infrastructure and a willingness to invest in advanced testing technologies that provide long-term analytical benefits and compliance with regulatory requirements.
Market Intelligence Brief:
Brazil's market expansion benefits from diverse food safety demand, including laboratory modernization in São Paulo and Rio de Janeiro, agricultural testing system upgrades, and government quality programs that increasingly incorporate analytical testing solutions for food safety applications. The country maintains a 9.0% CAGR through 2035, driven by rising food safety awareness and increasing adoption of test kit technology benefits, including precise detection capabilities and reduced analytical error capabilities.
Market dynamics focus on cost-effective test kit solutions that balance advanced analytical features with affordability considerations important to Brazilian testing providers. Growing laboratory infrastructure creates steady demand for modern analytical systems in new testing facilities and laboratory equipment modernization projects.
Strategic Market Considerations:
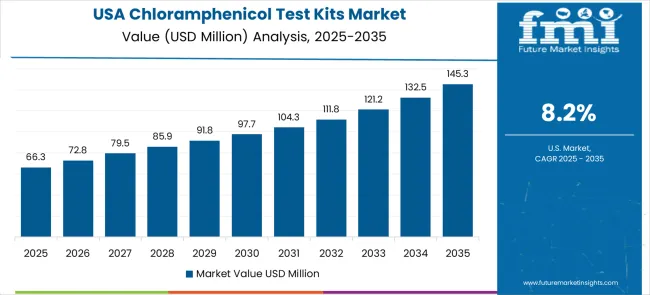
The USA market prioritizes advanced test kit features, including precision detection algorithms and integration with comprehensive laboratory information systems that manage analytical testing, quality control, and compliance applications through unified analytical platforms. The country is projected to show an 8.2% CAGR through 2035, driven by regulatory modernization under laboratory equipment upgrades and federal agency demand for reliable analytical testing systems. American laboratories prioritize testing effectiveness with test kits, delivering consistent analytical assistance through advanced detection algorithms and regulatory compliance capabilities.
Technology deployment channels include major analytical technology contractors, specialized laboratory equipment suppliers, and federal programs that support professional installation for complex testing applications. Laboratory platform integration capabilities with established analytical systems expand market appeal across diverse testing requirements seeking detection accuracy and regulatory compliance benefits.
Performance Metrics:
Japan demonstrates steady market development with a 6.5% CAGR through 2035, distinguished by laboratories' preference for high-quality test kit systems that integrate seamlessly with existing analytical equipment and provide reliable long-term operation in specialized testing applications. The market prioritizes advanced features, including precision detection algorithms, method validation, and integration with comprehensive analytical platforms that reflect Japanese laboratory expectations for technological sophistication and testing excellence.
In London, Manchester, and other analytical centers, British laboratories and testing facilities are implementing advanced test kit systems to enhance detection capabilities and support quality decision-making that aligns with regulatory standards and the requirements of analytical devices. The UK market is expected to demonstrate constant growth with a 7.3% CAGR through 2035, driven by food safety modernization programs and laboratory equipment upgrades that prioritize advanced analytical tools for testing and compliance applications. British analytical facilities are prioritizing test kit systems that provide precise detection capabilities while maintaining compliance with regulatory requirements and minimizing analytical error risks, particularly important in food safety and pharmaceutical applications.
Market expansion benefits from regulatory procurement programs that mandate analytical capabilities in laboratory equipment specifications, creating steady demand across the UK's testing sectors where analytical effectiveness and regulatory compliance represent critical requirements. The regulatory framework supports test kit adoption through analytical device standards and laboratory technology requirements that promote advanced detection systems aligned with national analytical capabilities.
Strategic Market Indicators:
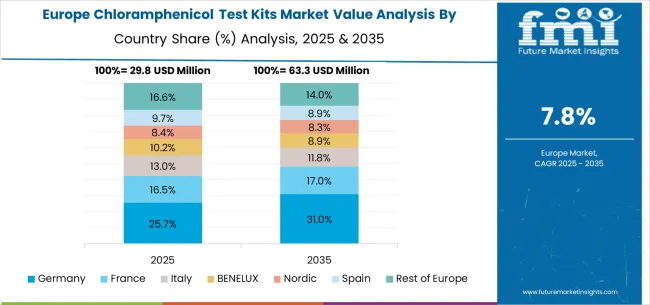
The chloramphenicol test kits market in Europe is projected to grow from USD 32.8 million in 2025 to USD 74.9 million by 2035, registering a CAGR of 8.6% over the forecast period. Germany is expected to maintain its leadership position with a 28.5% market share in 2025, rising slightly to 29.2% by 2035, supported by its advanced analytical infrastructure and major laboratory centers, including Munich and Berlin.
The United Kingdom follows with a 21.8% share in 2025, projected to reach 22.1% by 2035, driven by comprehensive food safety modernization programs and analytical technology development initiatives. France holds a 16.3% share in 2025, expected to increase to 16.7% by 2035 through specialized analytical applications and regulatory standardization requirements. Italy commands a 12.4% share in 2025, rising to 12.6% by 2035, while Spain accounts for 9.8% in 2025, increasing to 10.1% by 2035. BENELUX markets together represent 6.2% in 2025, moving to 6.4% by 2035, supported by innovation-friendly regulatory frameworks and industry-academic collaboration. The Nordic countries account for 3.1% in 2025, marginally increasing to 3.2% by 2035, with demand fueled by progressive analytical systems and early adoption of evidence-based testing. The Rest of Europe moderates from 1.9% in 2025 to 1.7% by 2035, as larger core markets capture a greater share of investment, analytical research, and adoption of advanced testing protocols.
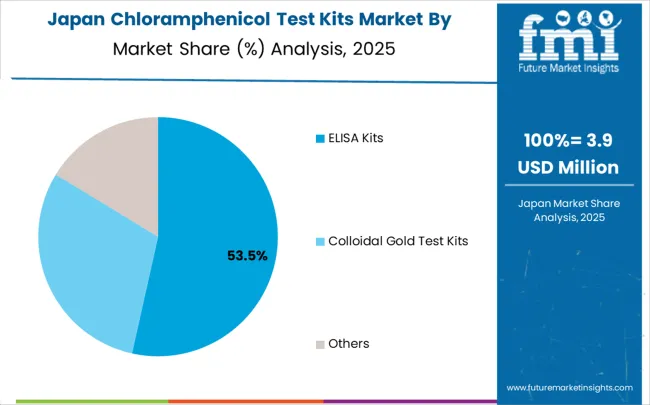
In Japan, the market prioritizes ELISA Kits deployment systems, which capture the dominant share of laboratory and testing facility installations through advanced features, including quantitative detection capabilities and seamless integration with existing analytical information technology infrastructure. Japanese laboratories prioritize reliability, detection accuracy, and long-term operational excellence, creating demand for ELISA systems that provide flexible analytical capabilities and adaptive processing control based on testing requirements and sample complexity scenarios. Colloidal gold test kits maintain a secondary market position primarily in specialized applications and rapid testing installations where speed requirements meet regulatory compliance without laboratory connectivity features.
Market Characteristics:
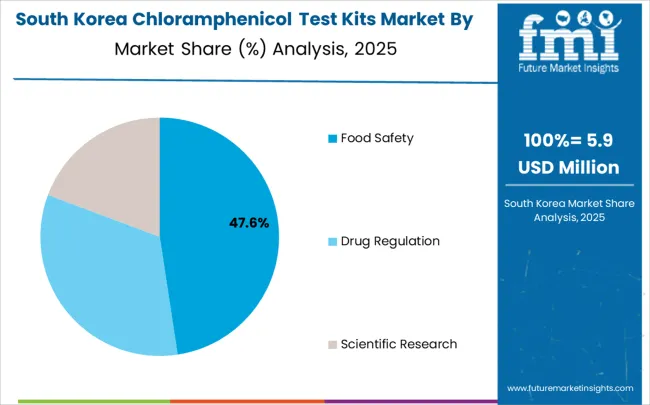
In South Korea, the market structure favors international analytical technology companies, including Creative Diagnostics, PerkinElmer, and Gold Standard Diagnostics Kassel, which maintain dominant positions through comprehensive product portfolios and established laboratory procurement networks supporting both testing systems and analytical facilities. These providers offer integrated solutions combining advanced test kit systems with professional installation services and ongoing technical support that appeal to Korean laboratories seeking reliable analytical testing systems. Local analytical technology contractors and system integrators capture a moderate market share by providing localized service capabilities and competitive pricing for standard laboratory installations. At the same time, domestic manufacturers focus on specialized applications and cost-effective solutions tailored to Korean analytical characteristics.
Channel Insights:
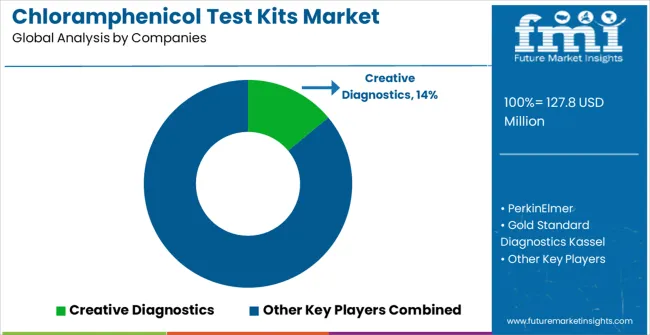
The market operates with moderate concentration, featuring approximately 15-20 meaningful participants, where leading companies control roughly 60-65% of the global market share through established analytical technology relationships and comprehensive testing platform portfolios. Competition prioritizes advanced detection capabilities, system reliability, and laboratory platform integration rather than price-based rivalry.
Market Leaders encompass Creative Diagnostics, PerkinElmer, Gold Standard Diagnostics Kassel, R-Biopharm Nederland, and FireGene, which maintain competitive advantages through extensive analytical technology expertise, global laboratory contractor networks, and comprehensive system integration capabilities that create customer switching costs and support premium pricing. These companies leverage decades of analytical technology experience and ongoing research investments to develop advanced test kit systems with precision detection algorithms and method validation features.
Technology Challengers include MyBioSource, Cusabio Technology, United States Biological, Novatein Biosciences, and Creative Bioarray, which compete through specialized analytical focus and innovative detection interfaces that appeal to laboratory customers seeking advanced testing accuracy capabilities and analytical flexibility. These companies differentiate through rapid technology development cycles and specialized testing application focus.
Regional Specialists feature companies like Bio-Techne, Demeditec Diagnostics, IBL, and Abbexa, which focus on specific geographic markets and specialized applications, including pharmaceutical testing systems and integrated quality platforms. Market dynamics favor participants that combine reliable detection algorithms with advanced analytical software, including precision residue analysis and automatic compliance reporting capabilities. Competitive pressure intensifies as traditional laboratory technology contractors expand into analytical testing systems. At the same time, specialized detection companies challenge established players through innovative testing solutions and cost-effective platforms targeting specialized analytical segments.
Chloramphenicol test kits represent essential analytical tools for detecting antibiotic residues across food safety, pharmaceutical regulation, and scientific research applications, with the market projected to grow from USD 127.8 million in 2025 to USD 291.7 million by 2035 at an 8.6% CAGR. As regulatory requirements intensify globally and laboratory automation advances, scaling this market requires coordinated action across government agencies, international standards organizations, analytical technology companies, laboratory operators, food industry stakeholders, and investment partners to address the complex challenges of detection sensitivity, method validation, regulatory harmonization, and laboratory integration.
How Government Agencies Could Strengthen Analytical Infrastructure?
Regulatory Standardization: Establish unified analytical methods and acceptance criteria for chloramphenicol test kits across major regulatory jurisdictions, enabling mutual recognition of test results and reducing validation complexity for laboratories serving multiple markets. Create fast-track approval pathways for validated test kits that meet international performance standards.
Laboratory Capacity Building: Fund modernization programs for public health laboratories, food safety testing centers, and customs inspection facilities to implement advanced test kit technologies. Provide training grants for laboratory personnel on proper test kit usage, data interpretation, and quality assurance procedures.
Method Validation Support: Establish national reference laboratories that conduct comprehensive validation studies comparing test kit performance against official analytical methods, providing regulatory acceptance data and performance benchmarks for different food matrices and sample types.
Data Integration Systems: Develop centralized databases that collect test kit results from regulatory testing programs, enabling real-time monitoring of chloramphenicol contamination trends, risk assessment, and targeted enforcement actions across different geographic regions and food categories.
Procurement Standardization: Create framework agreements for test kit procurement that establish performance specifications, quality requirements, and cost benchmarks, enabling smaller laboratories to access validated testing technologies through consolidated purchasing programs.
How International Standards Bodies Could Enable Market Harmonization?
Performance Standards Development: Establish globally recognized performance criteria for different test kit technologies (ELISA, colloidal gold, others), including sensitivity thresholds, cross-reactivity specifications, and matrix interference limits that ensure consistent detection capabilities across diverse applications.
Validation Protocol Standardization: Create comprehensive validation guidelines that specify required studies for different test kit types, including accuracy, precision, robustness, and stability testing protocols that enable regulatory acceptance across multiple jurisdictions.
Quality Management Standards: Develop specialized quality management requirements for test kit manufacturers, including good manufacturing practices, batch release testing, and post-market surveillance programs that ensure consistent product performance and reliability.
Laboratory Accreditation Requirements: Establish specific competency requirements for laboratories using different test kit technologies, including personnel qualifications, equipment specifications, and quality control procedures that ensure reliable results across different testing environments.
Inter-laboratory Comparison Programs: Organize global proficiency testing schemes that evaluate laboratory performance using different test kit technologies, providing objective assessment of testing capabilities and identifying areas for improvement across various regions and laboratory types.
How Analytical Technology Companies Could Advance Innovation?
Next-Generation Detection Platforms: Develop advanced ELISA systems with enhanced sensitivity, reduced matrix interference, and automated processing capabilities that achieve sub-ppb detection limits while maintaining cost-effectiveness for routine testing applications.
Digital Integration Solutions: Create comprehensive laboratory information management interfaces that seamlessly integrate test kit results with existing quality management systems, enabling automated reporting, trend analysis, and regulatory compliance documentation.
Multi-Analyte Capabilities: Design test kit platforms capable of simultaneous detection of chloramphenicol and related antibiotics, reducing testing time and costs while providing comprehensive antibiotic residue screening in single analytical workflows.
Point-of-Care Innovations: Develop portable test kit systems with smartphone connectivity and cloud-based data management that enable field testing, real-time result sharing, and immediate decision-making for food safety and regulatory applications.
Automation Integration: Create test kit formats compatible with existing laboratory automation systems, including liquid handling robots and automated plate readers, enabling high-throughput testing capabilities for large-scale monitoring programs.
How Laboratory Service Providers Could Optimize Implementation?
Method Integration Strategies: Develop comprehensive testing workflows that integrate different test kit technologies based on sample types, sensitivity requirements, and regulatory specifications, optimizing analytical efficiency while maintaining result reliability.
Quality Assurance Programs: Implement robust quality control systems that include regular proficiency testing, method validation maintenance, and performance monitoring to ensure consistent test kit performance across different analytical conditions.
Training and Certification: Establish specialized training programs for laboratory personnel on proper test kit usage, troubleshooting procedures, and result interpretation, ensuring consistent analytical performance across different operators and testing scenarios.
Data Management Systems: Deploy advanced laboratory information systems that capture, analyze, and report test kit results in formats required by different regulatory agencies, enabling efficient compliance reporting and trend analysis.
Customer Support Services: Provide comprehensive technical support to food industry clients, including sample preparation guidance, result interpretation assistance, and regulatory compliance consulting that facilitates effective test kit utilization.
How Food Industry Stakeholders Could Strengthen Supply Chain Monitoring?
Supplier Qualification Programs: Implement mandatory testing requirements for agricultural suppliers using validated test kits, providing training and technical support to ensure consistent testing practices and accurate result reporting across diverse supplier networks.
Risk-Based Testing Strategies: Develop data-driven testing protocols that prioritize high-risk suppliers, seasonal patterns, and historical contamination trends, optimizing test kit utilization while maintaining comprehensive monitoring coverage.
Supply Chain Integration: Create integrated testing programs that combine farm-level screening, processing facility monitoring, and finished product verification using appropriate test kit technologies for each control point.
Traceability Enhancement: Implement digital systems that link test kit results with product batch records, supplier information, and distribution data, enabling rapid response to contamination incidents and targeted product recalls.
Industry Collaboration Networks: Establish information-sharing platforms that enable anonymous reporting of test kit results and contamination trends, creating industry-wide visibility into chloramphenicol risks and testing effectiveness.
How Investment Partners Could Accelerate Market Development?
Technology Innovation Funding: Support research and development initiatives focused on breakthrough detection technologies, including biosensor integration, artificial intelligence-enhanced result interpretation, and novel analytical chemistries that could significantly improve test kit performance.
Manufacturing Scale-Up Capital: Finance production capacity expansion for validated test kit technologies, particularly in regions with growing regulatory testing requirements, ensuring adequate supply to meet projected market demand growth.
Laboratory Infrastructure Investment: Provide funding for laboratory modernization projects that implement advanced test kit technologies, automation systems, and digital integration capabilities, accelerating market adoption through improved testing infrastructure.
Market Access Financing: Support test kit manufacturers seeking regulatory approvals in multiple jurisdictions, reducing barriers to international market expansion and accelerating global availability of validated testing solutions.
Consolidation Strategies: Back strategic acquisitions that create integrated analytical service platforms, combining test kit manufacturing with laboratory services, regulatory consulting, and data management capabilities.
Asia-Pacific Leadership: Leverage China's 11.6% CAGR and India's 10.8% growth by supporting technology transfer initiatives, manufacturing capacity development, and regulatory framework enhancement that maintains cost advantages while achieving international quality standards.
European Excellence: Build on Germany's 9.9% growth and established analytical leadership by promoting advanced detection technologies, method validation programs, and comprehensive training initiatives that set global benchmarks for test kit performance and laboratory practices.
Americas Integration: Support Brazil's 9.0% growth and USA technological advancement by facilitating cross-border technology partnerships, harmonizing analytical methods, and developing integrated laboratory networks that strengthen food safety monitoring across both North and South American markets.
Emerging Market Development: Address infrastructure limitations in developing regions through targeted investment in laboratory capacity, technical training programs, and technology transfer initiatives that enable effective test kit implementation while building local analytical capabilities.
Public-Private Partnerships: Establish collaborative programs between government agencies, test kit manufacturers, and laboratory service providers that combine regulatory expertise, technological innovation, and operational experience to accelerate market development and improve testing effectiveness.
Academic Research Collaboration: Support university research programs focused on novel detection methods, analytical chemistry innovations, and validation studies that advance test kit technology while training the next generation of analytical professionals.
International Development Programs: Create programs that transfer test kit technology and analytical expertise to developing countries, building global food safety capacity while expanding market opportunities for established test kit manufacturers and laboratory service providers.
| Item | Value |
|---|---|
| Quantitative Units | USD 127.8 million |
| Product Type | ELISA Kits, Colloidal Gold Test Kits, Others |
| Application | Food Safety, Drug Regulation, Scientific Research |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, the USA, the UK, Japan, and 25+ additional countries |
| Key Companies Profiled | Creative Diagnostics, PerkinElmer, Gold Standard Diagnostics Kassel, R-Biopharm Nederland, FireGene, MyBioSource, Cusabio Technology, United States Biological, Novatein Biosciences |
| Additional Attributes | Dollar sales by product type and application categories, regional adoption trends across North America, Europe, and Asia-Pacific, competitive landscape with analytical technology providers and testing specialists, laboratory preferences for detection accuracy and system reliability, integration with laboratory information systems and analytical workflows, innovations in detection algorithms and sensitivity analysis, and development of automated solutions with enhanced scalability and accessibility capabilities. |
The global chloramphenicol test kits market is estimated to be valued at USD 127.8 million in 2025.
The market size for the chloramphenicol test kits market is projected to reach USD 291.7 million by 2035.
The chloramphenicol test kits market is expected to grow at a 8.6% CAGR between 2025 and 2035.
The key product types in chloramphenicol test kits market are elisa kits, colloidal gold test kits and others.
In terms of application, food safety segment to command 49.6% share in the chloramphenicol test kits market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Chloramphenicol Rapid Test Strip Market Size and Share Forecast Outlook 2025 to 2035
Test and Measurement Equipment Market Size and Share Forecast Outlook 2025 to 2035
Testosterone Test Market Size and Share Forecast Outlook 2025 to 2035
Test rig Market Size and Share Forecast Outlook 2025 to 2035
Test and Measurement Sensors Market Size and Share Forecast Outlook 2025 to 2035
Testing, Inspection & Certification Market Growth – Trends & Forecast 2025 to 2035
Testosterone Booster Industry Analysis by Component, Source, Distribution Channels and Regions 2025 to 2035
Testosterone Injectable Market
Test Tube Market
Testliner Market
Testicular Cancer Treatment Market
Intestinal Health Pet Dietary Supplement Market Size and Share Forecast Outlook 2025 to 2035
Intestinal Pseudo-Obstruction Treatment Market - Trends, Growth & Forecast 2025 to 2035
Intestinal Fistula Treatment Market Growth - Demand & Innovations 2025 to 2035
5G Testing Market Size and Share Forecast Outlook 2025 to 2035
AB Testing Software Market Size and Share Forecast Outlook 2025 to 2035
RF Test Equipment Market Size and Share Forecast Outlook 2025 to 2035
5G Testing Equipment Market Analysis - Size, Growth, and Forecast 2025 to 2035
5G Tester Market Growth – Trends & Forecast 2019-2027
RF Tester Market Growth – Trends & Forecast 2019-2027

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA