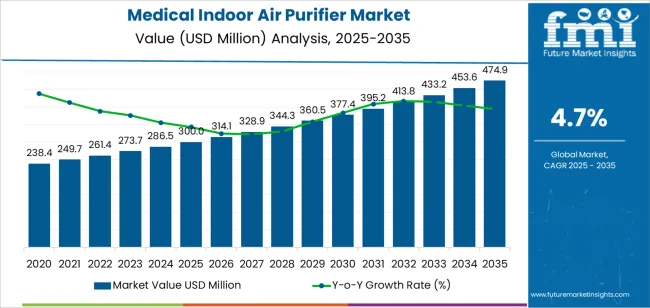
The global medical indoor air purifier market is forecasted to grow from USD 300.0 million in 2025 to approximately USD 474.9 million by 2035, recording an absolute increase of USD 174.9 million over the forecast period.Demand for medical indoor air purifiers is being reinforced by infection prevention priorities, facility upgrades, and stricter clinical air-quality protocols. Purchases are concentrated in hospitals, where floor-standing systems are favored for operating rooms, ICUs, and multi-bed wards because airflow coverage and filter capacity can be scaled to changing occupancy needs.
Clinics are adopting wall-mounted units to preserve floor space while meeting particulate and pathogen reduction targets in exam and treatment rooms. Product roadmaps are centered on HEPA H13–H14 filtration paired with activated carbon, UV-C or UV-A LED chambers, and real-time IAQ sensing that feeds auto-response modes, filter life prediction, and compliance logging. Buyers are requiring verified CADR at specified noise levels, certification under medical or cleanroom standards, and clear total cost of ownership models that include filter supply and service cycles. Barriers tend to arise from capital budgeting, retrofit constraints in older buildings, and the staffing needed for preventive maintenance. North America and Europe are setting the pace on specification rigor and traceable performance records, while Asia Pacific is posting faster unit growth with new hospital builds and private network expansion.
Technical advancements in filtration technologies and air purification systems enable more sophisticated infection control protocols that support diverse healthcare applications and extended operational requirements. Major medical facilities increasingly prioritize solutions that offer consistent performance across different clinical scenarios while meeting stringent safety and efficacy standards. The market benefits from established clinical evidence supporting air purification effectiveness combined with ongoing research into enhanced filtration approaches. Supply chain considerations favor systems that provide reliable performance and simplified maintenance protocols for healthcare facility management teams operating under demanding environmental control conditions.
| Metric | Value |
|---|---|
| Market Value (2025) | USD 300.0 million |
| Market Forecast Value (2035) | USD 474.9 million |
| Forecast CAGR (2025-2035) | 4.7% |
| HEALTHCARE INFRASTRUCTURE EXPANSION | INFECTION CONTROL REQUIREMENTS | REGULATORY & QUALITY STANDARDS |
|---|---|---|
|
|
|
| Category | Segments Covered |
|---|---|
| By Installation Type | Floor-Standing Type, Wall-Mounted Type, Others |
| By Application | Hospital, Clinic, Others |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |

| Segment | 2025 to 2035 Outlook |
|---|---|
| Floor-Standing Type |
|
| Wall-Mounted Type |
|
| Others |
|
| Segment | 2025 to 2035 Outlook |
|---|---|
| Hospital |
|
| Clinic |
|
| Others |
|
| DRIVERS | RESTRAINTS | KEY TRENDS |
|---|---|---|
|
|
|
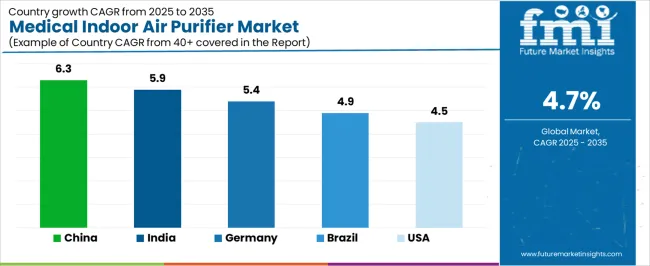
| Country | CAGR (2025-2035) |
|---|---|
| China | 6.3% |
| India | 5.9% |
| Germany | 5.4% |
| Brazil | 4.9% |
| United States | 4.5% |
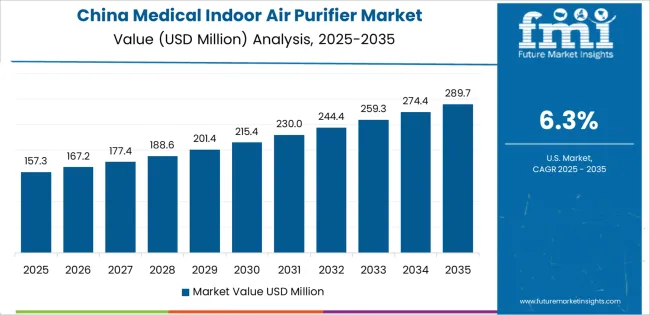
Revenue from medical indoor air purifiers in China is expected to expand at a 6.3% CAGR, reaching significant market value by 2035, driven by expanding hospital construction programs and comprehensive healthcare facility modernization. This is creating substantial opportunities for air purification system providers across major medical centers, specialty hospitals, and community healthcare facilities. The country's rapid healthcare infrastructure development and expanding medical quality standards are creating significant demand for medical-grade air purification systems. Major healthcare institutions and hospital chains are establishing comprehensive infection control protocols to support large-scale patient care operations and meet growing demand for clinical-grade air quality.
Revenue from medical indoor air purifiers in India is projected to grow at a 5.9% CAGR, reaching USD 149.0 million by 2035, supported by healthcare system expansion and comprehensive medical facility development. This is creating sustained demand for air quality control systems across diverse hospitals and emerging medical centers. The country's growing healthcare infrastructure and expanding quality standards are driving demand for systems that provide consistent clinical performance while supporting cost-effective procurement requirements. Healthcare institutions and hospital administrators are investing in infection control technologies to support growing patient volumes and quality improvement initiatives.
Demand for medical indoor air purifiers in Germany is expected to grow at a 5.4% CAGR, reaching USD 314.1 million by 2035, supported by the country's leadership in healthcare facility management and advanced infection control protocols. This requires sophisticated air purification systems for diverse clinical environments and complex medical procedures. German healthcare institutions are implementing high-quality air quality control systems that support clinical best practices, operational efficiency, and comprehensive safety standards. The market is characterized by a focus on clinical excellence, regulatory compliance, and adherence to stringent medical device and facility standards.
Revenue from medical indoor air purifiers in Brazil is growing at a 4.9% CAGR, reaching USD 238.5 million by 2035, driven by healthcare system modernization programs and increasing medical facility development. This creates sustained opportunities for system providers serving both public healthcare facilities and private hospital networks. The country's expanding healthcare infrastructure and growing medical device market are creating demand for air purification systems that support diverse clinical requirements while maintaining quality standards. Healthcare institutions and facility managers are developing procurement strategies to support operational efficiency and infection control protocol compliance.
Revenue from medical indoor air purifiers in the United States is expected to expand at a 4.5% CAGR, reaching USD 249.7 million by 2035, driven by clinical innovation excellence and established healthcare infrastructure supporting advanced infection control protocols and comprehensive facility management systems. The country's mature healthcare market and stringent regulatory environment are creating demand for air purification systems that support clinical performance and regulatory compliance standards. Healthcare institutions and facility management organizations are maintaining comprehensive quality protocols to support diverse medical facility requirements.

The medical indoor air purifier market in Europe is projected to grow from USD 86.6 million in 2025 to USD 135.9 million by 2035, registering a CAGR of 4.6% over the forecast period. Germany is expected to maintain its leadership position with a 29.2% market share in 2025, declining slightly to 28.7% by 2035, supported by its advanced healthcare infrastructure and major hospital networks including Charité and university medical centers.
France follows with a 21.8% share in 2025, projected to reach 22.3% by 2035, driven by comprehensive hospital modernization programs and infection control initiatives. The United Kingdom holds a 18.4% share in 2025, expected to maintain stability at 18.2% by 2035 with continued NHS facility upgrade investments. Italy commands a 15.6% share, while Spain accounts for 14.0% in 2025. The Rest of Europe region is anticipated to maintain its collective share at approximately 12% through 2035, with increasing adoption in Nordic countries and Central European medical facilities implementing enhanced air quality protocols.
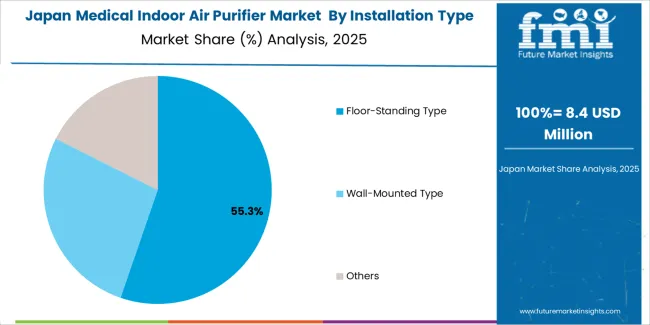
Japanese medical indoor air purifier operations reflect the country's exacting quality standards and sophisticated healthcare facility protocols. Major hospitals and medical centers maintain rigorous supplier qualification processes that often exceed international standards, requiring extensive documentation, performance testing, and facility validation that can take 12-18 months to complete. This creates high barriers for new suppliers but ensures consistent quality that supports optimal patient safety outcomes.
The Japanese market demonstrates unique air quality requirements, with significant emphasis on ultra-fine particulate filtration and precise airflow control specifications tailored to established clinical protocols. Regulatory oversight through the Pharmaceuticals and Medical Devices Agency emphasizes comprehensive quality management and performance verification requirements that surpass most international standards.
Supply chain management focuses on relationship-based partnerships rather than purely transactional procurement. Japanese healthcare facilities typically maintain long-term supplier relationships spanning years, with contract negotiations emphasizing performance consistency and technical support over price competition.

South Korean medical indoor air purifier operations reflect the country's advanced healthcare infrastructure and technology-oriented facility management approach. Major medical centers including Seoul National University Hospital and Samsung Medical Center drive sophisticated air purification system procurement strategies, establishing relationships with international suppliers to secure consistent quality and regulatory compliance for healthcare facilities.
The Korean market demonstrates particular strength in integrating air purification systems with smart building technologies, with leading hospitals combining air quality monitoring with facility management systems and automated environmental controls. Regulatory frameworks emphasize medical device safety and performance standards, with Ministry of Food and Drug Safety requirements for comprehensive documentation and quality assurance.
Supply chain efficiency remains critical given Korea's mix of domestic manufacturing and international equipment procurement. Healthcare institutions increasingly pursue long-term contracts with both local and international manufacturers to ensure reliable access while managing regulatory compliance requirements.
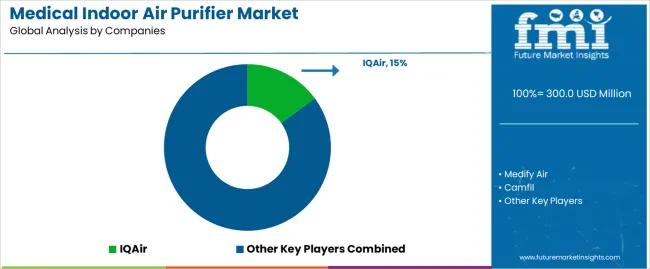
Profit pools concentrate in medical-grade filtration technology development and certified healthcare facility systems where clinical validation, regulatory compliance, and facility management relationships command sustained margins. Value migrates from residential-grade air purifiers to application-specific medical systems with documented infection control efficacy and comprehensive regulatory approvals. Several competitive archetypes define market dynamics: established air quality companies leveraging healthcare facility relationships and regulatory expertise; specialized medical air purification developers with proprietary filtration technologies and clinical validation partnerships; commercial HVAC manufacturers expanding into medical-grade solutions; and international distributors serving regional healthcare networks with established product portfolios. Switching costs remain substantial given facility qualification requirements, infection control protocol integration, and facility management training needs, stabilizing supplier relationships for proven systems. Market entry barriers include regulatory approval timelines, clinical validation requirements, and established procurement relationships within healthcare facility networks. Consolidation occurs selectively as larger facility equipment companies acquire specialized air purification technology platforms to expand healthcare portfolio offerings. Digital facility management influences commodity equipment procurement while specialized medical systems remain relationship-driven given clinical performance criticality. Strategic imperatives include securing healthcare facility partnerships with comprehensive technical support, establishing multi-regional regulatory approvals and performance certifications, and developing application-specific systems addressing infection control needs across diverse clinical environments and facility types.
| Stakeholder Type | Primary Advantage | Repeatable Plays |
|---|---|---|
| Established air quality companies | Regulatory expertise, healthcare facility relationships | Multi-product portfolios, clinical validation infrastructure, global distribution networks |
| Specialized medical air purification developers | Proprietary filtration technologies, infection control partnerships | Novel filtration development, hospital collaborations, targeted clinical studies |
| Commercial HVAC manufacturers | Facility system integration, maintenance networks | Building system compatibility, service infrastructure, facility management partnerships |
| International distributors | Regional market access, local regulatory knowledge | Multi-country certifications, healthcare facility relationships, technical support capabilities |
| Items | Values |
|---|---|
| Quantitative Units | USD 300.0 million |
| Product | Floor-Standing Type, Wall-Mounted Type, Others |
| Application | Hospital, Clinic, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | United States, Germany, China, Brazil, India, and other 40+ countries |
| Key Companies Profiled | IQAir, Medify Air, Camfil, AAF International, MedicAir, Aerobiotix, ISO-Aire, Atlas Copco, airinspace, AirQuality, Cairn Technology, MayAir Group, Omni CleanAir, RGF Environmental, Fantech, Air Impurities Removal Systems, Rensair, TION Ltd, CFULL, Oransi |
| Additional Attributes | Dollar sales by installation type/application, regional demand (NA, EU, APAC), competitive landscape, hospital vs. clinic adoption, filtration technology innovation, and smart monitoring systems driving infection control improvement, facility management efficiency, and clinical safety standards |
The global medical indoor air purifier market is estimated to be valued at USD 300.0 million in 2025.
The market size for the medical indoor air purifier market is projected to reach USD 474.9 million by 2035.
The medical indoor air purifier market is expected to grow at a 4.7% CAGR between 2025 and 2035.
The key product types in medical indoor air purifier market are floor-standing type, wall-mounted type and others.
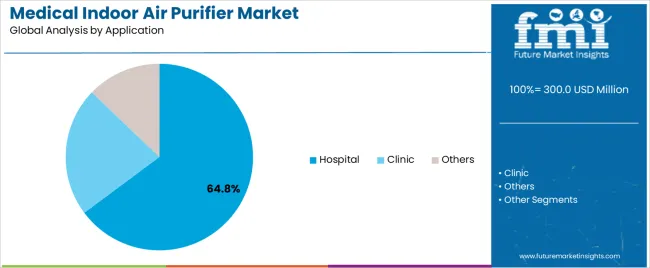
In terms of application, hospital segment to command 64.8% share in the medical indoor air purifier market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Medical Eye Shield Film Market Size and Share Forecast Outlook 2025 to 2035
Medical Far Infrared Therapy Device Market Size and Share Forecast Outlook 2025 to 2035
Medical Latex Protective Suit Market Size and Share Forecast Outlook 2025 to 2035
Medical Activated Carbon Dressing Market Size and Share Forecast Outlook 2025 to 2035
Medical Coated Roll Stock Market Size and Share Forecast Outlook 2025 to 2035
Medical Billing Outsourcing Market Size and Share Forecast Outlook 2025 to 2035
Medical Pressure Mapping System Market Size and Share Forecast Outlook 2025 to 2035
Medical Exoskeleton Market Forecast Outlook 2025 to 2035
Medical Display Market Forecast and Outlook 2025 to 2035
Medical Spa Market Size and Share Forecast Outlook 2025 to 2035
Medical Face Shield Market Forecast and Outlook 2025 to 2035
Medical Robot Market Size and Share Forecast Outlook 2025 to 2035
Medical Nutrition Market Forecast and Outlook 2025 to 2035
Medical Wax Market Size and Share Forecast Outlook 2025 to 2035
Medical Specialty Bag Market Size and Share Forecast Outlook 2025 to 2035
Medical Plastics Market Size and Share Forecast Outlook 2025 to 2035
Medical Device Tester Market Size and Share Forecast Outlook 2025 to 2035
Medical Device Trays Market Size and Share Forecast Outlook 2025 to 2035
Medical Adhesives Market Size and Share Forecast Outlook 2025 to 2035
Medically Supervised Weight Loss Services Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA