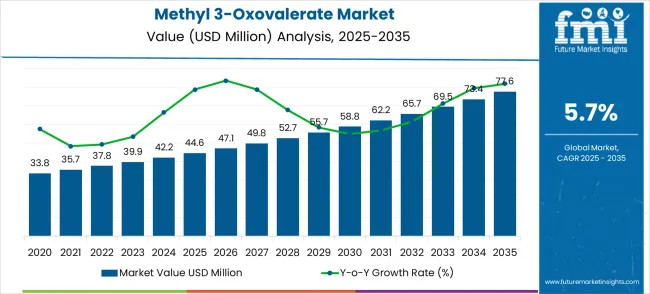
The Methyl 3-Oxovalerate market is anticipated to grow from USD 44.6 million in 2025 to USD 77.6 million by 2035, reflecting a CAGR of 5.7%. A breakpoint analysis reveals critical milestones in this growth trajectory. Between 2020 and 2024, the market experienced early adoption, with gradual increases from USD 33.8 million to USD 42.2 million. This phase represents selective usage in flavor, fragrance, and specialty chemical applications, where companies evaluated product efficacy and supply reliability. By 2025, reaching USD 44.6 million, the market crosses its first key threshold, indicating broader acceptance among industrial users and establishing a foundation for scaling adoption in the subsequent five years.
| Methyl 3-Oxovalerate Market | Value |
|---|---|
| Market Value (2025) | USD 44.6 million |
| Market Forecast Value (2035) | USD 77.6 million |
| Market Forecast CAGR | 5.7% |
From 2025 to 2030, the market enters a scaling phase, growing from USD 44.6 million to approximately USD 55.7 million. This period represents the next critical breakpoint where adoption accelerates across multiple sectors, including fine chemicals, pharmaceuticals, and fragrance intermediates. Production capacities expand, and distribution networks strengthen to meet increasing demand. By 2030, the market surpasses USD 55 million, reflecting its transition from niche applications to more widespread industrial use. This breakpoint highlights both volume growth and deeper penetration into end-user segments, signaling a more mature phase of market expansion and stronger revenue contribution from established players.
Between 2030 and 2035, the market reaches its consolidation phase, rising from USD 55.7 million to USD 77.6 million. This final breakpoint underscores the shift toward market maturity, with leading suppliers optimizing product portfolios, improving supply chain efficiency, and securing long-term contracts. By 2035, the market has achieved broader global adoption, with steady, predictable growth that aligns closely with the 5.7% CAGR. This phase reflects stable demand from established industrial sectors, minimal volatility, and continued incremental growth, establishing Methyl 3-Oxovalerate as a reliable revenue contributor within the specialty chemicals market.
Market expansion is being supported by the rapid increase in pharmaceutical manufacturing activities worldwide and the corresponding need for high-purity chemical intermediates that provide superior synthesis performance and reliable chemical properties. Modern pharmaceutical companies rely on consistent chemical quality and intermediate reliability to ensure optimal drug development including active pharmaceutical ingredient synthesis, complex organic reactions, and specialized chemical processes. Even minor purity variations can require comprehensive synthesis protocol adjustments to maintain optimal product quality and manufacturing performance.
The growing complexity of pharmaceutical synthesis requirements and increasing demand for high-purity chemical intermediates are driving demand for methyl 3-oxovalerate from certified manufacturers with appropriate purity capabilities and technical expertise. Pharmaceutical companies are increasingly requiring documented chemical purity and compound reliability to maintain synthesis quality and process effectiveness. Industry specifications and quality standards are establishing standardized synthesis procedures that require specialized chemical intermediates and precise manufacturing processes.
The methyl 3-oxovalerate market is entering a specialized growth phase driven by increasing pharmaceutical industry development, advancing organic synthesis methodologies, and rising demand for high-purity chemical intermediates. As pharmaceutical companies and research institutions across both developed and emerging markets seek chemical compounds that are high-purity, reliable, versatile, and regulatory-compliant, methyl 3-oxovalerate is evolving from a basic chemical intermediate to a strategic synthesis building block integrated with advanced pharmaceutical manufacturing, complex drug development, and specialized organic chemistry applications.
Rising pharmaceutical investments and research activities in Asia-Pacific, Latin America, and other emerging regions amplify demand, while manufacturers are advancing innovations in purification technology, quality control systems, and synthesis optimization. Pathways like pharmaceutical-grade purity, custom synthesis services, and regulatory compliance support promise strong margin uplift, especially in mature pharmaceutical markets. Geographic expansion and localization will capture volume growth, particularly where pharmaceutical manufacturing is developing or chemical synthesis capabilities are expanding. Regulatory pressures around pharmaceutical quality, chemical safety, and manufacturing standards provide structural market support.
The market is segmented by purity level, application, and region. By purity level, the market is divided into Purity 99% and others. Based on application, the market is categorized into organic synthesis, pharmaceutical intermediates, and others. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
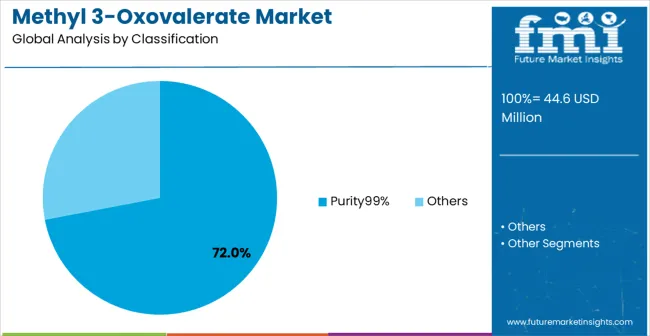
In 2025, the Purity 99% methyl 3-oxovalerate segment is projected to capture around 72% of the total market share, making it the leading purity category. This dominance is largely driven by the widespread adoption of high-purity chemical intermediates that deliver superior synthesis reliability and consistent chemical properties, catering to stringent pharmaceutical and research requirements for precise chemical specifications. The Purity 99% grade is particularly favored for its ability to provide optimal synthesis performance without introducing impurities that could compromise reaction outcomes, ensuring chemical reliability for diverse pharmaceutical manufacturing applications. Pharmaceutical manufacturers, research institutions, fine chemical producers, and specialty synthesis companies increasingly prefer this purity level, as it meets strict quality standards without imposing excessive purification costs or complex handling requirements.
The availability of well-established production processes, along with comprehensive quality control systems and technical support from leading chemical suppliers, further reinforces the segment's market position. Additionally, this purity category benefits from consistent demand across regions, as it is considered the optimal balance between chemical quality and cost-effectiveness for companies with varying synthesis requirements. The combination of proven reliability, consistent performance, and cost optimization makes Purity 99% methyl 3-oxovalerate a preferred choice, ensuring continued popularity in the chemical intermediates market. Advanced analytical testing and quality assurance protocols continue to drive adoption among quality-conscious manufacturers seeking reliable synthesis components.
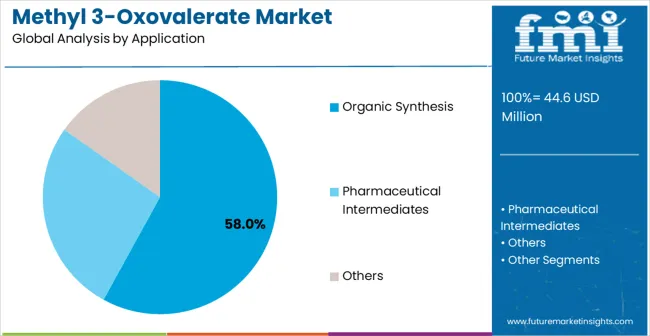
The organic synthesis segment is expected to represent 58% of methyl 3-oxovalerate demand in 2025, highlighting its position as the most significant application sector. This dominance stems from the unique chemical properties of methyl 3-oxovalerate in organic synthesis reactions, where its ketone functionality and ester group provide versatile reactivity for complex molecular transformations. Organic synthesis applications often feature sophisticated chemical reactions that demand reliable intermediate compounds throughout multi-step synthesis pathways, requiring precise chemical properties and consistent quality standards. Methyl 3-oxovalerate is particularly well-suited to organic synthesis due to its ability to participate in diverse chemical reactions including condensation reactions, reduction processes, and complex organic transformations, even during demanding synthesis conditions and extended reaction sequences. As pharmaceutical research and specialty chemical development continue to expand globally and emphasis on complex molecular synthesis increases, the demand for methyl 3-oxovalerate continues to rise.
The segment also benefits from heightened focus on advanced drug discovery and development programs, where researchers are increasingly utilizing specialized chemical intermediates and versatile synthesis building blocks as essential components of modern medicinal chemistry. With pharmaceutical companies investing in innovative drug development and synthetic methodology advancement, methyl 3-oxovalerate provides an essential solution to support high-performance organic synthesis capabilities. The growth of specialty pharmaceuticals, coupled with increased focus on complex natural product synthesis and advanced chemical research, ensures that organic synthesis applications will remain the largest and most stable demand driver for methyl 3-oxovalerate in the forecast period. Enhanced synthesis methodologies and improved reaction conditions continue to strengthen the segment's market position.
The Methyl 3-Oxovalerate market is advancing steadily due to increasing pharmaceutical industry development and growing recognition of specialized chemical intermediate advantages over conventional synthesis compounds. However, the market faces challenges including higher production costs compared to basic chemical intermediates, need for specialized handling and storage protocols, and varying regulatory compliance requirements across different pharmaceutical markets. Quality advancement efforts and purity optimization programs continue to influence product development and market adoption patterns.
The growing development of advanced pharmaceutical synthesis methodologies is enabling more sophisticated drug development processes with improved efficiency and selectivity characteristics. Enhanced synthesis technologies and optimized reaction conditions provide superior drug development capabilities while maintaining pharmaceutical quality standards. These technologies are particularly valuable for pharmaceutical companies who require reliable chemical intermediates that can support complex synthesis operations with consistent quality and predictable reaction outcomes throughout drug development processes.
Modern methyl 3-oxovalerate manufacturers are incorporating advanced analytical testing methods and quality control improvements that enhance product reliability and chemical consistency capabilities. Integration of advanced purity analysis systems and optimized manufacturing processes enables superior product quality and comprehensive batch-to-batch consistency. Advanced quality control features support pharmaceutical applications and research requirements while meeting various purity specifications and regulatory standards, including pharmaceutical-grade manufacturing, research applications, and specialized chemical synthesis processes.
The continuous expansion of pharmaceutical research and specialty chemical applications is driving demand for versatile chemical intermediates with specific reactivity profiles while maintaining optimal chemical stability. Advanced application development and specialized synthesis methodologies provide comprehensive chemical solutions that support innovative drug discovery, complex natural product synthesis, and advanced materials research requirements. These application innovations are particularly valuable for research institutions and pharmaceutical companies where chemical versatility and reliable performance are essential for successful synthesis projects and drug development programs.
| Country | CAGR (2025-2035) |
|---|---|
| China | 7.7% |
| India | 7.1% |
| Germany | 6.6% |
| Brazil | 6.0% |
| United States | 5.4% |
| United Kingdom | 4.8% |
| Japan | 4.3% |
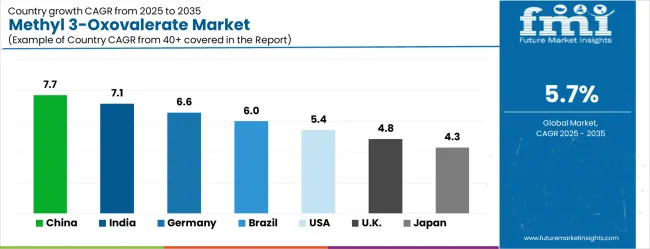
The methyl 3-oxovalerate market is growing rapidly, with China leading at a 7.7% CAGR through 2035, driven by strong pharmaceutical industry expansion and increasing demand for high-purity chemical intermediates. India follows at 7.1%, supported by rising pharmaceutical manufacturing capabilities and growing awareness of specialized chemical intermediate benefits. Germany grows steadily at 6.6%, integrating advanced chemical synthesis technologies into its established pharmaceutical manufacturing infrastructure. Brazil records 6.0%, emphasizing pharmaceutical industry development and chemical manufacturing modernization initiatives. The United States shows solid growth at 5.4%, focusing on advanced drug development and specialty chemical applications. The United Kingdom demonstrates steady progress at 4.8%, maintaining established pharmaceutical and chemical manufacturing applications. Japan records 4.3% growth, concentrating on quality enhancement and chemical synthesis optimization.
The report covers an in-depth analysis of 40+ countries top-performing countries are highlighted below.
Revenue from methyl 3-oxovalerate in China is projected to exhibit the highest growth rate with a CAGR of 7.7% through 2035, driven by rapid expansion of pharmaceutical manufacturing capabilities and increasing demand for high-purity chemical intermediates. The country's growing pharmaceutical industry and expanding chemical synthesis operations are creating significant demand for specialized organic compounds and reliable chemical intermediates. Major chemical manufacturers are establishing comprehensive production facilities to support the increasing requirements of pharmaceutical companies and research institutions across drug development and chemical synthesis applications.
Government initiatives supporting pharmaceutical industry development and chemical manufacturing modernization are facilitating establishment of advanced production capabilities and quality control systems, driving demand for sophisticated chemical intermediate technology throughout major pharmaceutical manufacturing regions and research centers. Pharmaceutical industry development programs are facilitating adoption of international quality standards that enhance chemical purity and synthesis reliability standards across manufacturing networks. China's focus on becoming a global pharmaceutical manufacturing hub is driving investment in high-quality chemical intermediate production capabilities.
The country's expanding research and development activities in pharmaceutical and biotechnology sectors are creating demand for specialized chemical building blocks. Government support for innovation in pharmaceutical manufacturing and chemical synthesis technologies is accelerating adoption of advanced intermediate compounds and supporting development of domestic chemical supply chains.
Revenue from methyl 3-oxovalerate in India is expanding at a CAGR of 7.1%, supported by increasing pharmaceutical manufacturing development and growing awareness of specialized chemical intermediate benefits. The country's expanding pharmaceutical industry and rising chemical synthesis capabilities are driving demand for high-purity organic compounds and reliable synthesis intermediates. Pharmaceutical companies and chemical manufacturers are gradually implementing advanced chemical intermediate technology to maintain manufacturing standards and synthesis reliability. Chemical industry sector growth and pharmaceutical manufacturing infrastructure development are creating opportunities for suppliers that can support diverse synthesis requirements and quality specifications.
Professional development and technical training programs are building expertise among chemical professionals, enabling effective utilization of specialized chemical intermediates that meet pharmaceutical manufacturing standards and synthesis quality requirements across research and production applications. India's position as a major pharmaceutical manufacturing destination is driving demand for reliable chemical intermediates that meet international quality standards. The country's growing contract manufacturing and research services sector requires consistent supply of specialized chemical compounds. Government initiatives supporting pharmaceutical exports and manufacturing excellence are promoting adoption of high-quality chemical intermediates that meet global pharmaceutical standards and regulatory requirements.
Demand for methyl 3-oxovalerate in Germany is projected to grow at a CAGR of 6.6%, supported by the country's emphasis on chemical manufacturing quality standards and advanced synthesis technology adoption. German pharmaceutical and chemical companies are implementing sophisticated chemical intermediate systems that meet stringent purity requirements and operational specifications. The market is characterized by focus on chemical quality, manufacturing reliability, and compliance with comprehensive pharmaceutical standards. Chemical industry investments are prioritizing advanced intermediate compounds that demonstrate superior purity performance and reliability while meeting German quality and regulatory standards. Professional certification programs are ensuring comprehensive technical expertise among chemical professionals, enabling specialized synthesis capabilities that support diverse pharmaceutical applications and chemical manufacturing requirements across regional networks. Germany's leadership in pharmaceutical and chemical manufacturing drives continuous investment in high-quality chemical intermediates and advanced synthesis technologies. The country's comprehensive regulatory framework and quality standards require sophisticated chemical compounds that meet strict purity and safety specifications. Integration with European pharmaceutical supply chains and manufacturing networks creates demand for reliable chemical intermediates with documented quality and traceability. The emphasis on sustainable chemical manufacturing and green chemistry principles is supporting adoption of efficient synthesis intermediates that reduce environmental impact.
Revenue from methyl 3-oxovalerate in Brazil is growing at a CAGR of 6.0%, driven by increasing pharmaceutical industry development and growing recognition of specialized chemical intermediate advantages. The country's expanding pharmaceutical manufacturing sector is gradually integrating advanced chemical intermediate technology to enhance synthesis capabilities and manufacturing reliability. Chemical companies and pharmaceutical manufacturers are investing in high-purity intermediate compounds to address evolving drug development requirements and manufacturing standards. Industry development is facilitating adoption of advanced chemical technologies that support comprehensive synthesis capabilities across pharmaceutical and chemical manufacturing regions.
Professional development programs are enhancing technical capabilities among chemical professionals, enabling effective chemical intermediate utilization that meets evolving pharmaceutical standards and synthesis requirements across manufacturing applications. Brazil's growing domestic pharmaceutical market and expanding manufacturing capabilities are creating demand for reliable chemical intermediates that support local drug production. The country's increasing participation in global pharmaceutical supply chains requires adherence to international quality standards and chemical specifications. Government initiatives supporting pharmaceutical industry growth and technology transfer are promoting adoption of advanced chemical intermediates and synthesis technologies that enhance manufacturing capabilities and product quality.
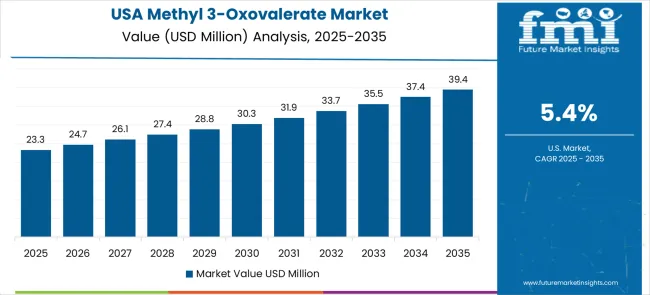
Demand for methyl 3-oxovalerate in the USA is expanding at a CAGR of 5.4%, driven by established pharmaceutical industries and growing emphasis on drug development innovation and specialty chemical applications. Large pharmaceutical companies and research institutions are implementing comprehensive chemical intermediate capabilities to serve diverse synthesis requirements and research applications. The market benefits from established chemical supply networks and advanced research programs that support various pharmaceutical development applications and chemical synthesis projects. Pharmaceutical industry leadership is enabling advanced compound utilization across multiple research categories, providing consistent innovation and comprehensive technical support throughout regional markets.
Professional research and development programs are building specialized technical expertise among chemical researchers, enabling effective chemical intermediate utilization that supports evolving pharmaceutical development requirements across metropolitan research centers. The USA market is characterized by strong emphasis on innovative drug discovery and advanced pharmaceutical research programs. Established biotechnology and pharmaceutical research infrastructure supports comprehensive chemical intermediate applications and specialized synthesis projects. The focus on personalized medicine and complex drug development is driving demand for versatile chemical building blocks that enable sophisticated molecular synthesis and drug design applications.
Demand for methyl 3-oxovalerate in the UK is projected to grow at a CAGR of 4.8%, supported by established pharmaceutical sectors and growing emphasis on chemical synthesis capabilities. British pharmaceutical companies and research institutions are implementing chemical intermediate equipment that meets industry quality standards and synthesis requirements. The market benefits from established pharmaceutical infrastructure and comprehensive research programs for drug development applications. Pharmaceutical investments are prioritizing advanced chemical compounds that support diverse synthesis applications while maintaining established quality and regulatory standards.
Professional development programs are building technical expertise among chemical professionals, enabling specialized chemical intermediate operation capabilities that meet evolving synthesis requirements and quality standards throughout research networks. The United Kingdom's established pharmaceutical research infrastructure and regulatory expertise support comprehensive chemical intermediate applications and quality assurance programs. The country's leadership in drug discovery and development creates demand for specialized chemical building blocks that meet international research standards. Integration with European pharmaceutical research networks and collaboration programs requires reliable chemical intermediates with documented purity and performance characteristics that support multinational research projects.
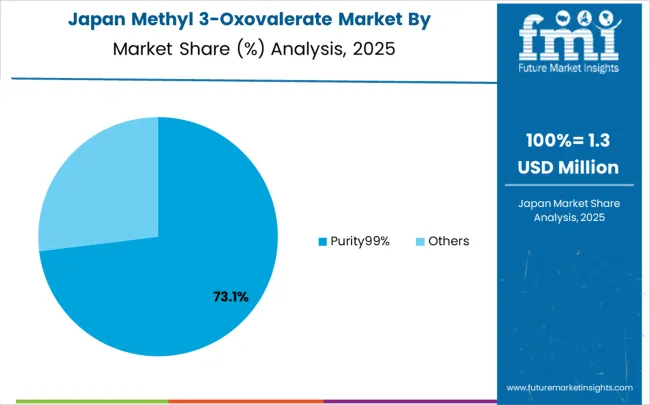
Revenue from methyl 3-oxovalerate in Japan is growing at a CAGR of 4.3%, driven by the country's focus on chemical synthesis innovation and quality enhancement applications. Japanese pharmaceutical and chemical companies are implementing advanced chemical intermediate systems that demonstrate superior purity reliability and synthesis efficiency. The market is characterized by emphasis on technological excellence, quality assurance, and integration with established pharmaceutical manufacturing workflows. Chemical industry investments are prioritizing innovative intermediate compounds that combine advanced synthesis technology with precision manufacturing while maintaining Japanese quality and reliability standards. Professional development programs are ensuring comprehensive technical expertise among chemical professionals, enabling specialized synthesis capabilities that support diverse pharmaceutical applications and chemical manufacturing requirements throughout metropolitan manufacturing networks.
Japan's commitment to pharmaceutical and chemical manufacturing excellence drives continuous innovation in chemical intermediate technology and synthesis optimization. The integration of advanced analytical methods and quality control systems with chemical synthesis processes requires sophisticated intermediate compounds with verified purity and performance specifications. The emphasis on precision manufacturing and technological advancement supports development of next-generation chemical intermediates with enhanced purity and improved synthesis applications that meet strict quality and performance requirements.
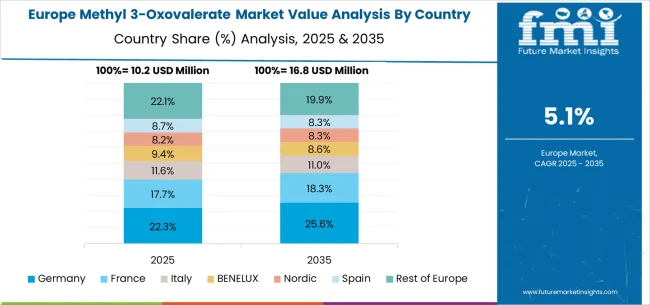
The methyl 3-oxovalerate market in Europe is projected to grow steadily from USD 14.3 million in 2025 to approximately USD 23.1 million by 2035, registering a CAGR of around 5.5% over the forecast period. Germany is expected to maintain its leadership with a 31.8% share in 2025, supported by its strong pharmaceutical manufacturing base and advanced chemical synthesis infrastructure. The United Kingdom follows with an 18.3% market share, driven by established pharmaceutical sectors and investments in chemical synthesis capabilities.
France holds 15.4% of the European market, benefiting from pharmaceutical research expansion and specialty chemical applications. Italy and Spain collectively represent 19.6% of regional demand, focusing on pharmaceutical development and high-purity intermediate manufacturing. The Rest of Europe accounts for 14.9% of the market, supported by growing pharmaceutical manufacturing and chemical intermediate production in Eastern European and Nordic countries.
The Methyl 3-Oxovalerate market is defined by competition among specialized chemical manufacturers, pharmaceutical intermediate suppliers, and fine chemical solution providers. Companies are investing in advanced synthesis technology development, purity optimization, quality control improvements, and comprehensive technical capabilities to deliver reliable, high-purity, and cost-effective chemical intermediate solutions. Strategic partnerships, technological innovation, and market expansion are central to strengthening product portfolios and market presence.
Matrix Fine Chemicals offers comprehensive methyl 3-oxovalerate solutions with established manufacturing expertise and pharmaceutical-grade chemical capabilities. Zhejiang Shengyu Chemical provides specialized organic intermediate production with focus on purity reliability and synthesis applications. Anhui Jinquan Biotechnology delivers advanced chemical synthesis solutions with emphasis on quality control and technical support. Lepu Medical Technology specializes in pharmaceutical intermediates with integrated chemical manufacturing capabilities.
Nanjing T.H Chemical offers professional-grade chemical intermediates with comprehensive quality assurance and regulatory compliance capabilities. Dudeli Chemical delivers established organic synthesis solutions with advanced purification technologies. Hangzhou Verychem Science and Technology provides specialized chemical manufacturing with focus on research applications and pharmaceutical intermediates, offering expertise in custom synthesis, analytical services, and quality control across global and regional market segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 44.6 million |
| Purity Level | Purity 99%, Others |
| Application | Organic Synthesis, Pharmaceutical Intermediates, Others |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | Matrix Fine Chemicals, Zhejiang Shengyu Chemical, Anhui Jinquan Biotechnology, Lepu Medical Technology, Nanjing T.H Chemical, Dudeli Chemical, Hangzhou Verychem Science and Technology |
| Additional Attributes | Dollar sales by purity level and application segment, regional demand trends across major markets, competitive landscape with established chemical manufacturers and emerging synthesis providers, customer preferences for different purity grades and chemical specifications, integration with pharmaceutical manufacturing systems and synthesis protocols, innovations in purification technology and quality control systems, and adoption of advanced analytical methods with enhanced chemical characterization capabilities for improved synthesis workflows. |
The global methyl 3-oxovalerate market is estimated to be valued at USD 44.6 million in 2025.
The market size for the methyl 3-oxovalerate market is projected to reach USD 77.6 million by 2035.
The methyl 3-oxovalerate market is expected to grow at a 5.7% CAGR between 2025 and 2035.
The key product types in methyl 3-oxovalerate market are purity99% and others.
In terms of application, organic synthesis segment to command 58.0% share in the methyl 3-oxovalerate market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Methylcobalamin Market Size and Share Forecast Outlook 2025 to 2035
Methylparaben Market Forecast and Outlook 2025 to 2035
Methyl Cyclohexane Market Size and Share Forecast Outlook 2025 to 2035
Methyl 2-Fluoro-3-Oxopentanoate Market Size and Share Forecast Outlook 2025 to 2035
Methyl 3-Methyl-2-Butenoate Market Size and Share Forecast Outlook 2025 to 2035
Methyl 2-Naphthyl Ether Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ionone Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ethyl Ketone Market Size and Share Forecast Outlook 2025 to 2035
Methyl Isobutyl Carbinol Market Size and Share Forecast Outlook 2025 to 2035
Methyl Ethyl Ketone Peroxide (MEKP) Market Size and Share Forecast Outlook 2025 to 2035
Methyl Oleate Market – Trends & Forecast 2025 to 2035
Methylamine Market Growth – Trends & Forecast 2024-2034
Methyl p-hydroxybenzoate Market
Methyl Cellulose Market
Methyl Isopropyl Ketone Market
Methylamines Market
Methylene Diphenyl Di-isocyanate Market
3-Methyl-3-penten-2-one (3M3P) Market Forecast and Outlook 2025 to 2035
Dimethyl Terephthalate Market Size and Share Forecast Outlook 2025 to 2035
Dimethylolpropionic Acid (DMPA) Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA