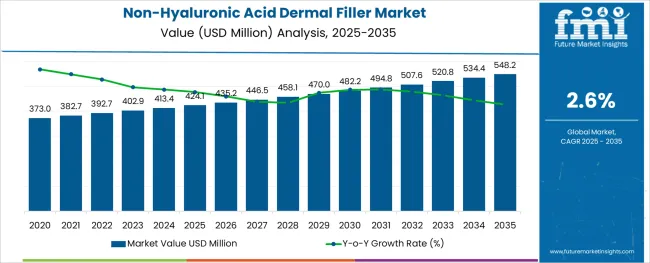
The Non-Hyaluronic Acid Dermal Filler Market is estimated to be valued at USD 424.1 million in 2025 and is projected to reach USD 548.2 million by 2035, registering a compound annual growth rate (CAGR) of 2.6% over the forecast period.
The non-hyaluronic acid dermal filler market is growing steadily as demand increases for diverse cosmetic solutions that cater to different patient needs. Rising awareness of aesthetic treatments and a growing emphasis on natural-looking volume restoration have driven interest in fillers beyond hyaluronic acid. Products like collagen-based fillers have been favored for their biocompatibility and ability to stimulate natural tissue regeneration.
Advances in formulation technologies have improved the safety and longevity of these fillers, making them attractive options for patients seeking effective and less invasive rejuvenation procedures. Clinics and aesthetic centers are expanding their offerings with these alternatives to address concerns such as allergies or previous adverse reactions to hyaluronic acid.
Future growth is expected to be supported by innovations in filler materials and increased adoption of aesthetic volume restoration treatments. Segmental growth is projected to be led by collagen as the dominant product type and aesthetic volume restoration as the primary application.
The market is segmented by Product Type and Application and region. By Product Type, the market is divided into Collagen, Hydroxylapatite, Polylactic Acid, Polyalkylimide, and Polymethyl-Methacrylate Microspheres. In terms of Application, the market is classified into Aesthetic Volume Restoration and Wrinkle Reduction. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, Balkan & Baltic Countries, Russia & Belarus, Central Asia, East Asia, South Asia & Pacific, and the Middle East & Africa.
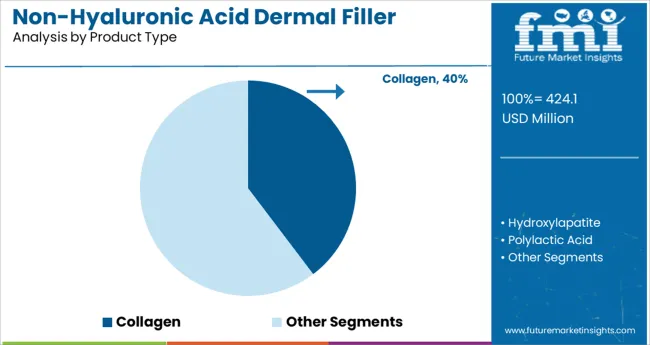
The collagen segment is projected to hold 39.7% of the non-hyaluronic acid dermal filler market revenue in 2025, positioning it as the leading product type. This growth is driven by collagen’s proven ability to improve skin texture and restore volume through natural tissue stimulation. Collagen fillers have been preferred for their compatibility with human tissue and reduced risk of adverse reactions.
Their use in minimally invasive procedures has expanded due to advances that extend their duration and enhance safety profiles. The segment benefits from sustained patient demand for natural and biocompatible aesthetic treatments.
Additionally, clinical practices have adopted collagen fillers as versatile tools for facial rejuvenation and correction of volume loss. As innovations continue to improve collagen-based products, this segment is expected to maintain its prominence.
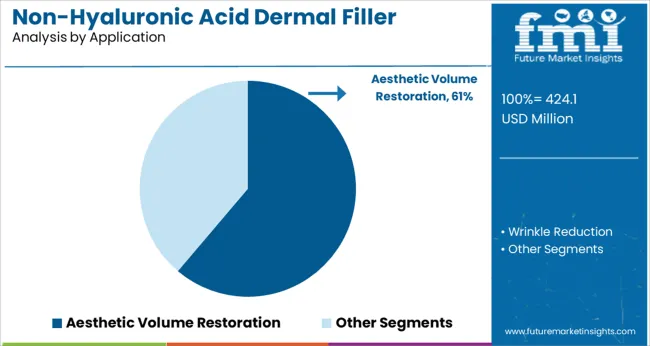
The aesthetic volume restoration segment is anticipated to contribute 61.2% of the market revenue in 2025, establishing itself as the primary application area. Growth in this segment stems from the rising popularity of non-surgical cosmetic procedures aimed at restoring facial volume lost due to aging or weight changes. Patients have increasingly sought effective treatments that provide immediate and natural-looking results without surgery.
Medical professionals have utilized dermal fillers extensively to target mid-face volume loss, cheek augmentation, and wrinkle reduction. The demand has been further driven by increasing acceptance of aesthetic treatments across different age groups and genders.
Continuous improvements in injection techniques and filler formulations have enhanced treatment outcomes, fueling the expansion of this application segment. As non-invasive cosmetic procedures become more mainstream, the aesthetic volume restoration segment is poised to sustain its market leadership.
The non-hyaluronic acid dermal filler market has received a lot of traction in the industry.
Increasing demand for surgical and medical cosmetic procedures, changing consumer preferences for less painful treatments, the preference of dermatologists for non-surgical treatments, and increased offerings for non-hyaluronic acid dermal fillers by the market leader are all factors driving demand for non-hyaluronic acid dermal fillers.
It is anticipated that greater advertising will be done in glossy magazines and hoardings by top manufacturers and distributors, encouraging people to become aware of aesthetic products and ultimately boosting the market for non-hyaluronic acid dermal fillers.
Nonetheless, improper training of professionals for non-hyaluronic acid dermal filler procedures and high physician services costs are factors inhibiting the growth of non-hyaluronic acid dermal fillers. In addition, reimbursement options are missing for minimally invasive treatments and procedures, which further hampers demand for non-hyaluronic acid dermal fillers.
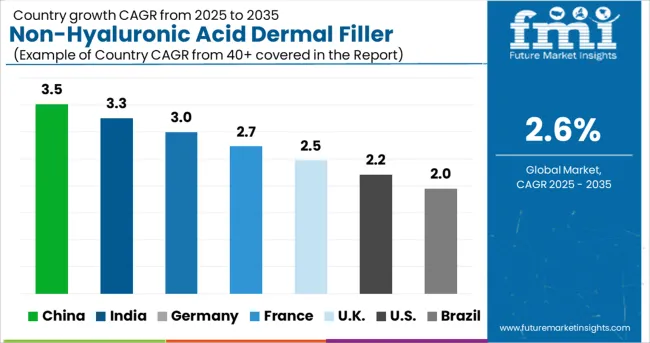
The market size of non-hyaluronic acid dermal fillers is classified geographically into seven key regions, including North America, Latin America, Western Europe, Eastern Europe, Asia Pacific, Japan, the Middle East, and Africa.
In North America, non-hyaluronic acid dermal fillers hold a 49.2% market value share because of increasing procedures associated with non-hyaluronic-based dermal fillers.
With continuous product launches by regional players, Western Europe is expected to account for the second-largest market value share at 22.8%.
Due to medical tourism for aesthetic procedures in ASEAN countries, APEJ can be seen as the fastest-growing region in terms of demand for non-hyaluronic acid dermal fillers, while MEA and Latin America hold the smallest market share by value due to shortages of skilled professionals.
How is the Start-up Ecosystem in the Non-Hyaluronic Acid Dermal Filler Market?
A successful FDA approval for Galderma's Restylane Kysse was received in May 2024. The product is approved for adults over 21 with indications of lip enhancement and correction of upper perioral rhytides (wrinkles around the top of the lips).
It was announced in November 2024 that Silk Medical Aesthetics and Evolved by Nature had completed a feasibility study for their all-natural dermal filler for nasolabial folds (NLFs) that had received FDA approval under the Investigational Device Exemption (IDE).
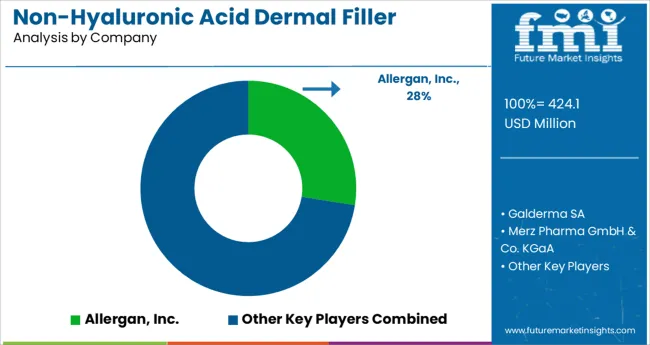
Some of the leading companies operating in the global non-hyaluronic acid dermal filler market include Allergan, Inc. Galderma SA, Merz Pharma GmbH & Co. KGaA, Suneva Medical Inc., Specialty European Pharma, Sinclair Pharma, Valeant Pharmaceuticals International Inc., LifeCell Corporation, Dr. Korman Laboratories Ltd., ForeverInject International Holdings Co. Limited.
For the promotion and marketing of non-hyaluronic acid dermal fillers, leading manufacturers are focusing on co-promotions and co-marketing strategies, forming strategic partnerships with international players in key target countries like Japan, South Korea, Thailand, Singapore, and UAE, and forming expansionary distribution agreements with dermatologists.
An FDA-approved Investigational Device Exemption (IDE) clinical trial of Silk Medical Aesthetics' all-natural dermal filler for nasolabial folds (NLFs) was conducted in November 2024 with Silver Medical Aesthetics and Evolved by Nature.
The USA Food and Drug Administration approved Restylane Kysse by Galderma in May 2024, indicated for lip augmentation and to lessen upper perioral rhytides (wrinkles around the lips).
| Report Attribute | Details |
|---|---|
| Growth Rate | CAGR of 2.6% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2020 to 2024 |
| Forecast Period | 2025 to 2035 |
| Quantitative Units | Revenue in million and CAGR from 2025-2035 |
| Report Coverage | Revenue Forecast, Volume Forecast, Company Ranking, Competitive Landscape, Growth Factors, Trends and Pricing Analysis |
| Segments Covered | Product Type, Application, Region |
| Regions Covered | North America; Latin America; Western Europe; Eastern Europe; Asia-Pacific; Japan; Middle East and Africa |
| Key Countries Profiled | USA, Canada, Brazil, Mexico, Germany, UK, France, Spain, Italy, Russia, Poland, Australia, New Zealand, China, India, Japan, GCC, South Africa, North Africa |
| Key Companies Profiled | Allergan, Inc.; Galderma SA; Merz Pharma GmbH & Co.; KGaA, Suneva Medical Inc.; Specialty European Pharma; Sinclair Pharma; Valeant Pharmaceuticals International Inc.; LifeCell Corporation; Dr. Korman Laboratories Ltd.; ForeverInject International Holdings Co. Limited. |
| Customization | Available Upon Request |
The global non-hyaluronic acid dermal filler market is estimated to be valued at USD 424.1 million in 2025.
It is projected to reach USD 548.2 million by 2035.
The market is expected to grow at a 2.6% CAGR between 2025 and 2035.
The key product types are collagen, hydroxylapatite, polylactic acid, polyalkylimide and polymethyl-methacrylate microspheres.
aesthetic volume restoration segment is expected to dominate with a 61.2% industry share in 2025.
Explore Similar Insights

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.