The ophthalmic diagnostic equipment market is expected to grow from USD 3.56 billion in 2025 to USD 5.02 billion by 2035. The market is poised to expand at a 3.5% CAGR during the forecast period. This growth is being propelled by the increasing global burden of eye disorders and the demand for early and precise diagnosis.
With the rise in conditions such as diabetic retinopathy, glaucoma, and age-related macular degeneration, healthcare providers are prioritizing investments in advanced diagnostic technologies. Instruments like fundus cameras, optical coherence tomography (OCT) systems, tonometers, and retinal ultrasound devices are becoming standard in ophthalmology practices, helping clinicians detect diseases at an early stage and prevent long-term vision loss.
The aging population, particularly in high-income regions, is also contributing to market expansion as routine eye health assessments become essential in geriatric care.
Additionally, the market is being driven by increased adoption of digital imaging and AI-integrated diagnostic platforms. Medical device companies are innovating compact, portable, and cloud-connected equipment that enables remote diagnostics, tele-ophthalmology, and mobile screening units.
These developments are especially impactful in underserved areas, where access to specialist care is limited. The integration of AI algorithms for pattern recognition and real-time analysis enhances clinical decision-making while reducing diagnostic errors.
As healthcare systems shift toward value-based models, demand for cost-effective and time-efficient diagnostics is growing, favoring technologies that offer quick turnaround and reliable data interpretation.
Furthermore, supportive government regulations and international health initiatives are contributing to the evolving landscape of the ophthalmic diagnostics industry. In the United States, FDA’s 510(k) pathway enables quicker market entry for upgraded versions of existing diagnostic tools, while Europe’s MDR framework enforces rigorous post-market surveillance, ensuring quality and safety.
merging economies are also building regulatory frameworks for AI-based diagnostics and are likely to accelerate approvals for cloud-enabled and mobile technologies. As a result, global manufacturers are investing in compliance, cybersecurity, and clinical validation to stay competitive.
The market is likely to benefit from these regulatory shifts as precision diagnostics become more accessible and scalable across both developed and developing regions.
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 3.56 billion |
| Industry Value (2035F) | USD 5.02 billion |
| CAGR (2025 to 2035) | 3.5% |
High surgical and treatment rates correlate directly with diagnostic capacity, from biometry and slit lamps to OCT and fundus cameras.
Scaling diagnostics is tied to meeting international service benchmarks, while fragmented procurement makes workload data the most reliable demand signal.
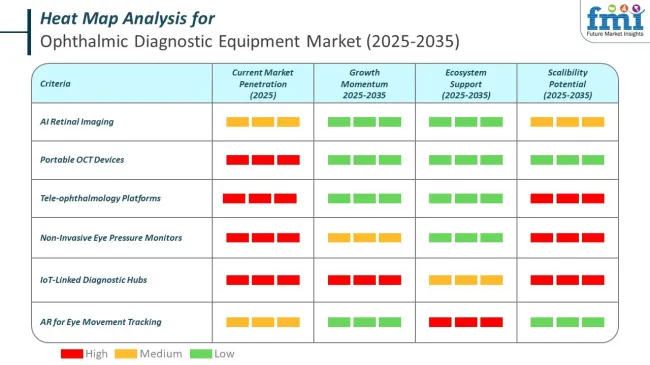
The market is segmented based on product, end user, and region. By product type, the market is divided into fundus cameras, retinal ultrasound imaging systems, refractors, slit lamps, perimeters, ophthalmoscopes, tonometers, optical coherence tomography (OCT), and corneal topography systems.
In terms of end user, it is segmented into hospitals, clinics, and ambulatory surgical centres. Regionally, the market is classified into North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, and the Middle East and Africa.
The optical coherence tomography (OCT) segment is projected to be the fastest-growing, registering a CAGR of 5.1% between 2025 and 2035, surpassing the overall market growth rate of 3.5%.
OCT’s non-invasive, high-resolution imaging makes it essential in detecting retinal pathologies, including macular degeneration, diabetic retinopathy, and glaucoma. Increasing reliance on early-stage diagnostics and follow-up assessments is strengthening demand among both general ophthalmologists and retina specialists.
The transition toward AI-integrated OCT systems that deliver real-time analysis and pattern recognition further enhances clinical utility. Portability and ease of use are making compact OCT devices attractive to outpatient settings, including ambulatory care. Other product categories such as fundus cameras, slit lamps, and tonometers continue to experience steady growth, supported by their indispensable role in general eye exams and routine screenings.
Perimeters, refractors, and corneal topography systems are also gaining usage in preoperative evaluations and specialty clinics, driven by procedural advancements in refractive and cataract surgery.
| Product | CAGR (2025-2035) |
|---|---|
| Optical Coherence Tomography (OCT) | 5.1% |
The ambulatory surgical centres (ASCs) segment is projected to grow at the fastest rate, with a CAGR of 4.8% from 2025 to 2035, supported by a systemic shift toward same-day procedures and outpatient eye care. ASCs are becoming increasingly important in managing cataract surgeries, glaucoma laser treatments, and minor retinal procedures, offering quicker recovery times and reduced hospital congestion.
The affordability, convenience, and efficient resource allocation offered by these centres are accelerating diagnostic equipment installations. Compact and automated diagnostic tools, including portable OCT systems and slit lamps, are being widely adopted to support volume-driven workflows. Meanwhile, hospitals continue to dominate in terms of total installations due to their role in complex diagnostics and surgical interventions.
Clinics are also contributing to segment growth, particularly in urban and semi-urban settings, where early-stage detection and preventive eye health services are being prioritized. Clinics are increasingly investing in mid-range imaging tools and tonometers to improve service delivery.
| End Use | CAGR (2025-2035) |
|---|---|
| Ambulatory Surgical Centres | 4.8% |
Rising Burden of Eye Diseases and Imaging Precision Accelerate Market Growth
The market is being driven by the increasing global prevalence of chronic and age-related eye diseases such as diabetic retinopathy, glaucoma, and age-related macular degeneration. Aging populations in North America, Europe, and East Asia are particularly vulnerable, prompting greater adoption of advanced imaging technologies like OCT and fundus cameras. The demand for non-invasive, early-stage diagnostics is rising in both clinical and outpatient settings.
Enhanced diagnostic accuracy, shorter scan times, and real-time image analysis through AI integration are improving clinical outcomes and expanding the utility of ophthalmic devices. Additionally, public health initiatives promoting routine vision screenings and avoidable blindness prevention are supporting diagnostic uptake, especially in urban and semi-urban areas.
With healthcare systems worldwide shifting toward value-based care and preventive medicine, high-resolution and cloud-enabled ophthalmic imaging platforms are becoming essential tools in modern eye care delivery.
High Equipment Costs and Limited Access to Trained Professionals Hinder Penetration
Despite growing demand, high capital expenditure and personnel limitations remain significant obstacles in many parts of the world. Advanced diagnostic equipment like OCT, retinal ultrasound systems, and perimeters require substantial upfront investments, limiting access for small clinics and rural healthcare providers.
The shortage of trained ophthalmic technicians and optometrists further compounds the issue, especially in low- and middle-income countries. Infrastructural gaps, such as unreliable power supply, insufficient IT connectivity, and lack of telehealth readiness, make equipment deployment and maintenance difficult. Additionally, inconsistent reimbursement policies and limited government funding for eye health restrict long-term planning for diagnostic expansion.
These barriers slow market penetration in underserved regions, despite the high burden of visual impairment. Bridging these challenges will require coordinated efforts between governments, global health organizations, and manufacturers offering cost-effective and scalable diagnostic solutions.
AI Integration and Decentralized Diagnostics Unlock Future Growth Potential
Expanding use of AI and the emergence of decentralized diagnostic models are creating substantial growth opportunities. AI-powered systems capable of detecting and classifying retinal diseases are gaining regulatory approvals and entering clinical practice across developed regions.
These tools reduce diagnostic workloads and improve access in areas with a shortage of ophthalmologists. Meanwhile, demand is growing for portable, cloud-connected devices that enable community-level screenings and home-based eye exams. Wearable imaging tools and smartphone-integrated cameras are allowing early intervention in school health programs and diabetic care networks.
Startups and medical device manufacturers are increasingly developing low-footprint, tele-ophthalmology-compatible platforms. Governments are also encouraging innovation through grants, digital health missions, and regulatory support for AI diagnostics. As preventive care becomes central to healthcare policy, players offering affordable, user-friendly, and AI-integrated systems will find ample room to scale and serve broader geographies.
Cybersecurity Vulnerabilities and Regulatory Gaps Threaten Innovation Adoption
As diagnostic devices become more digitally connected and reliant on AI, cybersecurity and regulatory fragmentation are emerging as serious threats. Systems that store and transmit patient images and health data are at risk of cyberattacks and unauthorized access, especially in cloud-enabled environments. Strict data privacy laws such as HIPAA in the USA and GDPR in Europe require vendors to maintain robust cybersecurity standards, encryption, and data governance protocols.
However, inconsistent regulatory frameworks across regions complicate compliance for multinational manufacturers and delay product approvals. Furthermore, ethical concerns regarding AI decision-making and lack of digital literacy among older patient populations can reduce trust and uptake. Failure to address these risks may result in reputational damage, loss of clinical confidence, or even legal liabilities.
To secure long-term adoption, companies must prioritize data integrity, transparency, and cross-border compliance in both hardware and software ecosystems.
| Countries | CAGR (2025-2035) |
|---|---|
| United States | 5.4% |
| United Kingdom | 4.5% |
| France | 7.2% |
| Germany | 6.1% |
| Japan | 2% |
The United States ophthalmic diagnostic equipment market is projected to grow at a CAGR of 5.4% from 2025 to 2035, driven by a surge in chronic eye disorders, growing demand for early-stage diagnostics, and significant investments in AI-integrated imaging platforms. With strong Medicare and private insurance support for routine eye exams, adoption of advanced devices like OCT, fundus cameras, and perimetry systems remains high across hospitals, specialty clinics, and outpatient centers.
USA based firms such as Johnson & Johnson Vision and Alcon, alongside global players like Carl Zeiss Meditec and Haag-Streit, are aggressively expanding R&D to deliver compact, high-throughput, and cloud-connected diagnostics. Tele-ophthalmology is rapidly gaining ground, supported by the FDA’s streamlined approvals for AI-based diagnostics.
Academic partnerships, venture funding for mobile diagnostics, and integration with EHR platforms continue to elevate the U.S. as a major hub for innovation. Rising consumer health awareness and remote patient monitoring initiatives are expected to maintain this momentum during the forecast period.
The UK ophthalmic diagnostic equipment market is projected to grow at a CAGR of 4.5% from 2025 to 2035. Market expansion is fueled by a rising geriatric population and increasing incidence of age-related vision disorders.
The National Health Service (NHS) is actively promoting early eye disease detection through community screening programs. Moreover, favorable reimbursement policies and growing investments in public healthcare infrastructure are encouraging hospitals and eye care clinics to upgrade their diagnostic systems.
Portable OCT and fundus imaging systems are seeing increased adoption, especially in mobile screening units and outpatient centers. Companies like Topcon Corporation and Nidek Co. Ltd have a strong distribution network across the UK, while collaborations between med-tech players and academic institutions are driving research in AI-assisted vision diagnostics. Despite Brexit-related supply disruptions, demand for early diagnostic intervention and AI-integrated equipment continues to support the UK’s robust market position.
The ophthalmic diagnostic equipment market in France is expected to expand at a CAGR of 7.2% between 2025 and 2035. Growth is driven by increased awareness around visual health, strong government support, and rapid technological adoption across healthcare providers.
France’s anti-waste and health modernization initiatives are boosting funding for digital medical devices, including non-invasive diagnostic platforms. OCT systems account for the largest share, while tonometers are emerging as the fastest-growing segment due to increased screening for glaucoma. Hospitals and ophthalmic centers are increasingly investing in AI-enhanced imaging systems to streamline diagnostics and patient management.
Domestic leaders such as EssilorLuxottica, alongside global players like Carl Zeiss Meditec, are expanding their R&D capabilities within France. The government’s support for innovation in med-tech, coupled with rising private insurance penetration and digital infrastructure upgrades, is expected to further enhance equipment adoption across urban and semi-urban regions.
Germany’s ophthalmic diagnostic equipment market is estimated to grow at a CAGR of 6.1% during the forecast period. As one of Europe’s largest healthcare markets, Germany benefits from a sophisticated infrastructure and high diagnostic demand driven by an aging population.
The country is also a manufacturing hub for precision-engineered diagnostic devices, with local companies like Carl Zeiss Meditec leading innovation in OCT and perimetry technologies. Hospitals and specialty eye clinics are modernizing their facilities with AI-enabled systems that enhance diagnostic accuracy and reduce patient wait times.
Government healthcare programs encourage preventative care, which boosts routine vision screenings and expands demand for equipment like fundus cameras and topography systems. Germany’s alignment with EU regulations also supports increased investment in tele-ophthalmology and cloud-based diagnostics, enabling broader patient coverage in rural regions. Continued export growth and rising domestic procurement are expected to keep Germany at the forefront of the European ophthalmic diagnostics landscape.
Japan’s ophthalmic diagnostic equipment market is forecast to grow at a modest CAGR of 2.0% from 2025 to 2035. The country’s aging demographic remains the primary driver for vision diagnostics, with conditions like cataracts, macular degeneration, and glaucoma increasingly prevalent among elderly populations.
Japan is home to several leading ophthalmic equipment manufacturers including Topcon Corporation and Nidek Co. Ltd, both of which dominate domestic distribution and contribute to exports. Government-led initiatives to digitize healthcare and strengthen home-based care delivery are expanding the role of portable diagnostic devices. However, market maturity and relatively slow regulatory changes have slightly restrained growth compared to other Asia-Pacific countries. There is also growing interest in integrating AI with OCT systems and mobile retinal imaging platforms to support early intervention.
Although Japan’s market is stable and highly automated, further acceleration is likely to depend on reforms in reimbursement systems and increased adoption of cloud-based diagnostic technologies.
The competitive landscape of the market is moderately consolidated, featuring a blend of global leaders and specialized innovators competing on technological precision, integration of AI, and accessibility.
Key players such as Carl Zeiss Meditec AG, Topcon Corporation, and NIDEK Co., Ltd. dominate with comprehensive diagnostic portfolios, spanning OCT systems, fundus cameras, tonometers, and perimeters. These companies are leveraging AI and cloud-based connectivity to improve diagnostic efficiency and broaden access to vision care. In contrast, regional specialists and Tier 2 players like Optovue, Canon Inc., and Haag-Streit AG focus on niche product enhancements, compact imaging systems, and cost-effective solutions for mid-sized clinics. Startups are emerging with innovations in wearable diagnostics and mobile screening devices.
Entry barriers such as stringent FDA and MDR approvals, high R&D costs, and reliance on specialized personnel limit new competition. As demand grows for decentralized and smart diagnostics, strategic partnerships, regulatory agility, and scalable product development remain critical success factors in an increasingly tech-driven and competitive industry.
Carl Zeiss Meditec AG continues to lead the market through precision imaging and data-driven platforms. Its CIRRUS HD-OCT and FORUM systems integrate AI-based analysis and cloud accessibility, offering real-time diagnostics and seamless workflow integration.
Zeiss is actively expanding in emerging markets by introducing lower-footprint diagnostic stations suitable for outpatient settings. Topcon Corporation maintains its global stronghold via multi-functional tools like the Triton swept-source OCT and Harmony software that allows interoperability across diagnostic devices.
These offerings support retina specialists, optometrists, and mobile screening programs alike. NIDEK Co., Ltd. remains a preferred provider in Asia and Latin America, focusing on durability, user-friendliness, and affordability.
European players such as Haag-Streit AG and Canon Inc. are pushing advancements in modular systems and hybrid cameras. Haag-Streit’s Octopus perimetry tools are valued for glaucoma detection, while Canon’s CX-1 hybrid digital camera bridges traditional and digital imaging.
Smaller disruptors like Optos, iCare, and Remidio are advancing compact fundus cameras and portable visual field testers, targeting teleophthalmology and rural eye care programs. Strategic alliances with AI developers and EMR providers are also emerging as key to long-term growth, particularly in value-based healthcare environments.
Recent Ophthalmic Diagnostic Equipment Industry News
| Attribute | Details |
|---|---|
| Current Total Market Size (2025) | USD 3.56 billion |
| Projected Market Size (2035) | USD 5.02 billion |
| CAGR (2025 to 2035) | 3.5% |
| Base Year for Estimation | 2024 |
| Historical Period | 2020-2024 |
| Projections Period | 2025-2035 |
| Report Parameter | Revenue in USD billion/volume in units |
| By Product | Fundus Cameras, Retinal Ultrasound Imaging Systems, Refractors, Slit Lamps, Perimeters, Ophthalmoscopes, Tonometer, Optical Coherence Tomography (OCT), and Corneal Topography Systems |
| By End User | Hospitals, Clinics, and Ambulatory Surgical Centres |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, South Asia and Pacific, East Asia, Middle East and Africa |
| Countries Covered | United States, United Kingdom, France, Germany, Japan |
| Key Players | TOPCON CORPORATION, ZEISS International, Ellex, Quantel Medical, NIDEK CO.LTD., HAAG-STREIT GROUP, Halma plc, Coburn Technologies Inc., Kowa Company Ltd., Carl Zeiss Meditec AG |
| Additional Attributes | Dollar sales by value, market share analysis by region, and country-wise analysis |
The global market is expected to reach USD 5.02 billion by 2035, growing from USD 3.56 billion in 2025, at a CAGR of 3.5% during the forecast period.
The optical coherence tomography (OCT) segment is projected to grow at the fastest pace, registering a CAGR of 5.1%, due to its non-invasive, high-resolution imaging capabilities and increasing integration with AI for early-stage diagnostics.
Ambulatory Surgical Centres are the fastest-growing end-use segment, driven by the rising demand for outpatient procedures and the adoption of compact diagnostic tools that support efficient and same-day eye care services.
Key drivers include the growing prevalence of chronic eye diseases, aging populations, rising demand for early and accurate diagnostics, and the shift toward tele-ophthalmology and AI-based diagnostic platforms.
Top companies include Carl Zeiss Meditec AG, Topcon Corporation, NIDEK Co., Ltd., Canon Inc., Haag-Streit Group, and EssilorLuxottica, all of which are advancing innovation in imaging systems, AI diagnostics, and portable solutions.
Table 1: Global Market Value (US$ Million) Forecast by Region, 2019 to 2034
Table 2: Global Market Volume (Units) Forecast by Region, 2019 to 2034
Table 3: Global Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 4: Global Market Volume (Units) Forecast by Product, 2019 to 2034
Table 5: Global Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 6: Global Market Volume (Units) Forecast by End User, 2019 to 2034
Table 7: North America Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 8: North America Market Volume (Units) Forecast by Country, 2019 to 2034
Table 9: North America Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 10: North America Market Volume (Units) Forecast by Product, 2019 to 2034
Table 11: North America Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 12: North America Market Volume (Units) Forecast by End User, 2019 to 2034
Table 13: Latin America Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 14: Latin America Market Volume (Units) Forecast by Country, 2019 to 2034
Table 15: Latin America Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 16: Latin America Market Volume (Units) Forecast by Product, 2019 to 2034
Table 17: Latin America Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 18: Latin America Market Volume (Units) Forecast by End User, 2019 to 2034
Table 19: Western Europe Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 20: Western Europe Market Volume (Units) Forecast by Country, 2019 to 2034
Table 21: Western Europe Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 22: Western Europe Market Volume (Units) Forecast by Product, 2019 to 2034
Table 23: Western Europe Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 24: Western Europe Market Volume (Units) Forecast by End User, 2019 to 2034
Table 25: Eastern Europe Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 26: Eastern Europe Market Volume (Units) Forecast by Country, 2019 to 2034
Table 27: Eastern Europe Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 28: Eastern Europe Market Volume (Units) Forecast by Product, 2019 to 2034
Table 29: Eastern Europe Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 30: Eastern Europe Market Volume (Units) Forecast by End User, 2019 to 2034
Table 31: South Asia and Pacific Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 32: South Asia and Pacific Market Volume (Units) Forecast by Country, 2019 to 2034
Table 33: South Asia and Pacific Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 34: South Asia and Pacific Market Volume (Units) Forecast by Product, 2019 to 2034
Table 35: South Asia and Pacific Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 36: South Asia and Pacific Market Volume (Units) Forecast by End User, 2019 to 2034
Table 37: East Asia Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 38: East Asia Market Volume (Units) Forecast by Country, 2019 to 2034
Table 39: East Asia Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 40: East Asia Market Volume (Units) Forecast by Product, 2019 to 2034
Table 41: East Asia Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 42: East Asia Market Volume (Units) Forecast by End User, 2019 to 2034
Table 43: Middle East and Africa Market Value (US$ Million) Forecast by Country, 2019 to 2034
Table 44: Middle East and Africa Market Volume (Units) Forecast by Country, 2019 to 2034
Table 45: Middle East and Africa Market Value (US$ Million) Forecast by Product, 2019 to 2034
Table 46: Middle East and Africa Market Volume (Units) Forecast by Product, 2019 to 2034
Table 47: Middle East and Africa Market Value (US$ Million) Forecast by End User, 2019 to 2034
Table 48: Middle East and Africa Market Volume (Units) Forecast by End User, 2019 to 2034
Figure 1: Global Market Value (US$ Million) by Product, 2024 to 2034
Figure 2: Global Market Value (US$ Million) by End User, 2024 to 2034
Figure 3: Global Market Value (US$ Million) by Region, 2024 to 2034
Figure 4: Global Market Value (US$ Million) Analysis by Region, 2019 to 2034
Figure 5: Global Market Volume (Units) Analysis by Region, 2019 to 2034
Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2024 to 2034
Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2024 to 2034
Figure 8: Global Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 9: Global Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 10: Global Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 11: Global Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 12: Global Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 13: Global Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 14: Global Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 15: Global Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 16: Global Market Attractiveness by Product, 2024 to 2034
Figure 17: Global Market Attractiveness by End User, 2024 to 2034
Figure 18: Global Market Attractiveness by Region, 2024 to 2034
Figure 19: North America Market Value (US$ Million) by Product, 2024 to 2034
Figure 20: North America Market Value (US$ Million) by End User, 2024 to 2034
Figure 21: North America Market Value (US$ Million) by Country, 2024 to 2034
Figure 22: North America Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 23: North America Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 26: North America Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 27: North America Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 28: North America Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 29: North America Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 30: North America Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 31: North America Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 32: North America Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 33: North America Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 34: North America Market Attractiveness by Product, 2024 to 2034
Figure 35: North America Market Attractiveness by End User, 2024 to 2034
Figure 36: North America Market Attractiveness by Country, 2024 to 2034
Figure 37: Latin America Market Value (US$ Million) by Product, 2024 to 2034
Figure 38: Latin America Market Value (US$ Million) by End User, 2024 to 2034
Figure 39: Latin America Market Value (US$ Million) by Country, 2024 to 2034
Figure 40: Latin America Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 41: Latin America Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 44: Latin America Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 45: Latin America Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 46: Latin America Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 48: Latin America Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 49: Latin America Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 50: Latin America Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 52: Latin America Market Attractiveness by Product, 2024 to 2034
Figure 53: Latin America Market Attractiveness by End User, 2024 to 2034
Figure 54: Latin America Market Attractiveness by Country, 2024 to 2034
Figure 55: Western Europe Market Value (US$ Million) by Product, 2024 to 2034
Figure 56: Western Europe Market Value (US$ Million) by End User, 2024 to 2034
Figure 57: Western Europe Market Value (US$ Million) by Country, 2024 to 2034
Figure 58: Western Europe Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 59: Western Europe Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 60: Western Europe Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 61: Western Europe Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 62: Western Europe Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 63: Western Europe Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 64: Western Europe Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 65: Western Europe Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 66: Western Europe Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 67: Western Europe Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 68: Western Europe Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 69: Western Europe Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 70: Western Europe Market Attractiveness by Product, 2024 to 2034
Figure 71: Western Europe Market Attractiveness by End User, 2024 to 2034
Figure 72: Western Europe Market Attractiveness by Country, 2024 to 2034
Figure 73: Eastern Europe Market Value (US$ Million) by Product, 2024 to 2034
Figure 74: Eastern Europe Market Value (US$ Million) by End User, 2024 to 2034
Figure 75: Eastern Europe Market Value (US$ Million) by Country, 2024 to 2034
Figure 76: Eastern Europe Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 77: Eastern Europe Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 78: Eastern Europe Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 79: Eastern Europe Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 80: Eastern Europe Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 81: Eastern Europe Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 82: Eastern Europe Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 83: Eastern Europe Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 84: Eastern Europe Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 85: Eastern Europe Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 86: Eastern Europe Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 87: Eastern Europe Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 88: Eastern Europe Market Attractiveness by Product, 2024 to 2034
Figure 89: Eastern Europe Market Attractiveness by End User, 2024 to 2034
Figure 90: Eastern Europe Market Attractiveness by Country, 2024 to 2034
Figure 91: South Asia and Pacific Market Value (US$ Million) by Product, 2024 to 2034
Figure 92: South Asia and Pacific Market Value (US$ Million) by End User, 2024 to 2034
Figure 93: South Asia and Pacific Market Value (US$ Million) by Country, 2024 to 2034
Figure 94: South Asia and Pacific Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 95: South Asia and Pacific Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 96: South Asia and Pacific Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 97: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 98: South Asia and Pacific Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 99: South Asia and Pacific Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 100: South Asia and Pacific Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 101: South Asia and Pacific Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 102: South Asia and Pacific Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 103: South Asia and Pacific Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 104: South Asia and Pacific Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 105: South Asia and Pacific Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 106: South Asia and Pacific Market Attractiveness by Product, 2024 to 2034
Figure 107: South Asia and Pacific Market Attractiveness by End User, 2024 to 2034
Figure 108: South Asia and Pacific Market Attractiveness by Country, 2024 to 2034
Figure 109: East Asia Market Value (US$ Million) by Product, 2024 to 2034
Figure 110: East Asia Market Value (US$ Million) by End User, 2024 to 2034
Figure 111: East Asia Market Value (US$ Million) by Country, 2024 to 2034
Figure 112: East Asia Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 113: East Asia Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 114: East Asia Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 115: East Asia Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 116: East Asia Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 117: East Asia Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 118: East Asia Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 119: East Asia Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 120: East Asia Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 121: East Asia Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 122: East Asia Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 123: East Asia Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 124: East Asia Market Attractiveness by Product, 2024 to 2034
Figure 125: East Asia Market Attractiveness by End User, 2024 to 2034
Figure 126: East Asia Market Attractiveness by Country, 2024 to 2034
Figure 127: Middle East and Africa Market Value (US$ Million) by Product, 2024 to 2034
Figure 128: Middle East and Africa Market Value (US$ Million) by End User, 2024 to 2034
Figure 129: Middle East and Africa Market Value (US$ Million) by Country, 2024 to 2034
Figure 130: Middle East and Africa Market Value (US$ Million) Analysis by Country, 2019 to 2034
Figure 131: Middle East and Africa Market Volume (Units) Analysis by Country, 2019 to 2034
Figure 132: Middle East and Africa Market Value Share (%) and BPS Analysis by Country, 2024 to 2034
Figure 133: Middle East and Africa Market Y-o-Y Growth (%) Projections by Country, 2024 to 2034
Figure 134: Middle East and Africa Market Value (US$ Million) Analysis by Product, 2019 to 2034
Figure 135: Middle East and Africa Market Volume (Units) Analysis by Product, 2019 to 2034
Figure 136: Middle East and Africa Market Value Share (%) and BPS Analysis by Product, 2024 to 2034
Figure 137: Middle East and Africa Market Y-o-Y Growth (%) Projections by Product, 2024 to 2034
Figure 138: Middle East and Africa Market Value (US$ Million) Analysis by End User, 2019 to 2034
Figure 139: Middle East and Africa Market Volume (Units) Analysis by End User, 2019 to 2034
Figure 140: Middle East and Africa Market Value Share (%) and BPS Analysis by End User, 2024 to 2034
Figure 141: Middle East and Africa Market Y-o-Y Growth (%) Projections by End User, 2024 to 2034
Figure 142: Middle East and Africa Market Attractiveness by Product, 2024 to 2034
Figure 143: Middle East and Africa Market Attractiveness by End User, 2024 to 2034
Figure 144: Middle East and Africa Market Attractiveness by Country, 2024 to 2034






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Ophthalmic Stereoscopic 3D Display Market Size and Share Forecast and Outlook 2025 to 2035
Ophthalmic Prefilled Injectables Market Size and Share Forecast Outlook 2025 to 2035
Ophthalmic Drug Packaging Market Forecast and Outlook 2025 to 2035
Ophthalmic Surgical Market Size and Share Forecast Outlook 2025 to 2035
Ophthalmic Injectable Market Size and Share Forecast Outlook 2025 to 2035
Ophthalmic Sprays Market Size and Share Forecast Outlook 2025 to 2035
Ophthalmic Tonometers Market – Demand, Growth & Forecast 2025 to 2035
Ophthalmic Lasers Market Analysis - Innovations & Forecast 2024 to 2034
Global Ophthalmic Combination Product Market Analysis – Size, Share & Forecast 2024-2034
Ophthalmic Eye Drop Market – Trends & Forecast 2024-2034
Ophthalmic Knives Market
Ophthalmic Viscosurgical Devices Market
Herpes Zoster Ophthalmicus Market Size and Share Forecast Outlook 2025 to 2035
Excimer and Femtosecond Ophthalmic Lasers Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Shipper Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Imaging Markers Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Imaging Services Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Vials Market Size and Share Forecast Outlook 2025 to 2035
Diagnostic Exosome Biomarkers Market Trends – Growth & Forecast 2025 to 2035
Diagnostic X-Ray System Market – Trends & Forecast 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA