The pharmaceutical grade magnesium sulfate market is projected to expand from USD 199.9 million in 2025 to USD 298.8 million by 2035. This growth reflects steady reliance on controlled production systems that ensure purity, stability, and pharmacopoeial compliance. The supply chain supporting this segment operates within defined quality boundaries due to its use in injectable and parenteral formulations, where contamination control and consistency are mandatory.
Raw material sourcing remains centered on natural magnesium ores, primarily derived from magnesite and dolomite. Producers in China, Europe, and the United States maintain regional extraction and conversion facilities that feed refined magnesium oxide into sulfate production units. Dependence on local mineral availability and the cost of purification chemicals influence pricing stability. Variations in ore grade and trace element content require extensive quality screening before pharmaceutical conversion. Supply risks are mitigated by vertical integration among major chemical groups that control both extraction and downstream refining.
Manufacturing processes involve crystallization, evaporation, and multiple purification cycles designed to achieve high-purity heptahydrate or anhydrous forms suitable for intravenous and oral preparations. The process sequence includes dissolution of magnesium oxide in sulfuric acid, controlled pH adjustment, and multi-stage filtration. Evaporation and crystallization conditions determine crystal size distribution and moisture content, which affect product solubility and stability during storage. Continuous process optimization and closed-system handling are used to reduce microbial exposure. Producers adhering to GMP standards maintain batch documentation and analytical traceability for each production run.
Logistics and distribution require controlled handling to preserve product integrity throughout the supply chain. Although magnesium sulfate does not require refrigeration, consistent temperature and humidity management are essential to prevent hydration state variation and caking. Packaging typically involves sealed polyethylene or laminated paper bags for solids and HDPE containers for solutions. Distribution channels are dominated by pharmaceutical wholesalers and hospital procurement networks that source from certified manufacturers. Export-oriented suppliers maintain validated shipping protocols and lot traceability in compliance with regulatory norms in North America, Europe, and the Asia-Pacific.
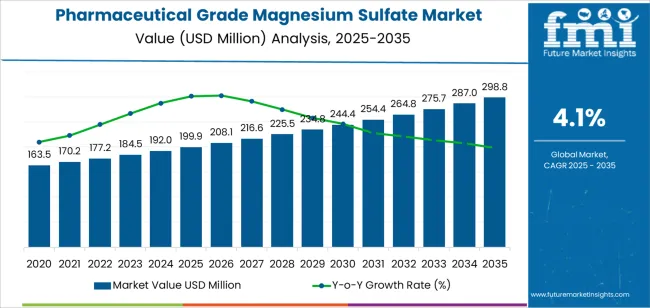
The pharmaceutical grade magnesium sulfate market demonstrates consistent growth phases with evolving therapeutic applications and quality requirements. Between 2025 and 2030, the pharmaceutical grade magnesium sulfate market progresses through its therapeutic expansion phase, growing from USD 199.9 million to USD 254.4 million with steady annual increments averaging 4.1% growth. This period showcases the transition from traditional obstetric applications to comprehensive therapeutic protocols with enhanced purity standards and integrated quality management becoming mainstream requirements.
The 2025-2030 phase adds USD 54.5 million to market value, representing 55% of total decade expansion. Market maturation factors include standardization of pharmaceutical manufacturing protocols, declining production costs for high-purity formulations, and increasing healthcare provider awareness of magnesium deficiency reaching critical recognition levels in clinical practice. Competitive landscape evolution during this period features established pharmaceutical chemical manufacturers like K+S and PQ Corporation expanding their therapeutic-grade portfolios while specialty producers focus on advanced purification technology and enhanced quality assurance capabilities.
From 2030 to 2035, market dynamics shift toward specialized therapeutic applications and global healthcare standardization, with growth continuing from USD 254.4 million to USD 298.8 million, adding USD 44.4 million or 45% of total expansion. This phase transition centers on precision dosing formulations, integration with digital healthcare platforms, and deployment across diverse medical scenarios, becoming standard rather than specialized applications. The competitive environment matures with focus shifting from basic pharmaceutical-grade capability to comprehensive therapeutic optimization systems and integration with hospital pharmacy management platforms.
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 199.9 million |
| Market Forecast (2035) ↑ | USD 298.8 million |
| Growth Rate ★ | 4.1% CAGR |
| Leading Purity Level → | Purity>99% |
| Primary Application → | Pregnancy-Related Diseases |
Pregnancy-related disease applications drive primary demand, supported by increasing maternal healthcare standards and obstetric care modernization requirements. Geographic expansion remains concentrated in developed markets with established pharmaceutical manufacturing infrastructure, while emerging economies show accelerating adoption rates driven by healthcare system development and rising clinical standards.
Market expansion rests on three fundamental shifts driving adoption across healthcare and pharmaceutical manufacturing sectors. First, pregnancy-related complications create compelling clinical demand through pharmaceutical grade magnesium sulfate that provides immediate therapeutic intervention for pre-eclampsia and eclampsia management, enabling hospitals to meet treatment protocols while maintaining patient safety and reducing maternal mortality rates. Second, hypomagnesemia recognition accelerates as healthcare facilities worldwide seek therapeutic compounds that complement traditional treatment approaches, enabling precise electrolyte management and metabolic balance that align with clinical guidelines and evidence-based protocols.
Third, digestive system disorder treatment drives adoption from gastroenterology specialists and hospital pharmacies requiring effective laxative solutions that enhance patient compliance while maintaining therapeutic outcomes during treatment regimens. However, growth faces headwinds from alternative compound availability that varies across pharmaceutical suppliers regarding magnesium oxide and citrate formulations, which may limit adoption in cost-sensitive healthcare markets. Quality control challenges also persist regarding impurity levels and batch consistency variations that may reduce efficacy in critical-care applications, which affect treatment outcomes and clinical reliability.
The pharmaceutical grade magnesium sulfate market represents a specialized yet essential pharmaceutical ingredient opportunity driven by expanding therapeutic applications, hospital modernization, and the need for superior quality standards in critical care processes. As healthcare facilities worldwide seek to achieve precise therapeutic dosing, ensure regulatory compliance, and integrate advanced pharmaceutical compounds with digital hospital platforms, magnesium sulfate is evolving from basic chemical compound to sophisticated therapeutic agent that ensures treatment efficacy and patient safety.
The convergence of maternal healthcare advancement, metabolic disorder recognition, and pharmaceutical quality enhancement creates sustained demand drivers across multiple medical segments. The pharmaceutical grade magnesium sulfate market's growth trajectory from USD 199.9 million in 2025 to USD 298.8 million by 2035 at a 4.1% CAGR reflects fundamental shifts in hospital treatment requirements and pharmaceutical quality optimization.
Geographic expansion opportunities are particularly pronounced in Asia-Pacific markets, where China (5.5% CAGR) and India (5.1% CAGR) lead through aggressive healthcare infrastructure development and pharmaceutical manufacturing expansion. The dominance of Purity>99% systems and pregnancy-related disease applications provides clear strategic focus areas, while emerging therapeutic applications and specialized formulation development open new revenue streams across diverse medical markets.
Strengthening the dominant Purity>99% segment through enhanced pharmaceutical compliance, superior therapeutic efficacy, automated production compatibility, and seamless integration with modern pharmaceutical manufacturing infrastructure. This pathway focuses on optimizing purification processes, improving batch consistency, extending quality assurance to comprehensive testing protocols, and developing specialized formulations for diverse therapeutic applications. Market leadership consolidation through advanced chemical processing, comprehensive regulatory certification, and quality management integration enables premium positioning while defending competitive advantages against lower-purity alternatives. Expected revenue pool: USD 42-68 million
Strategic expansion within pregnancy-related disease applications through advanced pre-eclampsia management, eclampsia treatment optimization, and comprehensive maternal care protocols for specialty obstetric facilities. This pathway addresses critical maternal health requirements, emergency treatment scenarios, and evidence-based obstetric practices with advanced pharmaceutical-grade formulations for demanding clinical standards. Premium positioning reflects therapeutic leadership, clinical expertise, and comprehensive safety capabilities while enabling access to hospital procurement programs and maternal healthcare networks. Expected revenue pool: USD 35-56 million
Expansion within hypomagnesemia prevention applications through specialized electrolyte management formulations, metabolic disorder treatment programs, and comprehensive therapeutic support for hospital pharmacy operations. This pathway encompasses precise dosing systems, therapeutic monitoring integration, cost-effective solutions, and compatibility with diverse clinical scenarios. Premium positioning reflects superior therapeutic outcomes, consistent efficacy, and comprehensive clinical support that enables modern hospital operations while facilitating integration with electronic health records and pharmacy management systems. Expected revenue pool: USD 28-45 million
Rapid healthcare infrastructure development across China (5.5% CAGR) and India (5.1% CAGR) creates substantial expansion opportunities through local manufacturing capabilities, hospital network partnerships, and comprehensive distribution development. Growing maternal healthcare awareness, pharmaceutical manufacturing advancement, and regulatory standardization initiatives drive sustained demand for pharmaceutical-grade compounds. Localization strategies reduce import costs, enable faster regulatory approval, and position companies advantageously for hospital procurement programs while accessing growing domestic markets requiring quality pharmaceutical ingredients. Expected revenue pool: USD 22-35 million
Development within digestive system disease applications through advanced laxative formulations, gastrointestinal treatment protocols, and specialized delivery systems for gastroenterology practices. This pathway encompasses controlled-release technologies, combination therapy compatibility, patient compliance enhancement, and comprehensive therapeutic optimization. Premium positioning through formulation innovation, clinical efficacy optimization, and gastrointestinal expertise creates opportunities for specialty pharmaceutical partnerships and therapeutic advancement. Expected revenue pool: USD 18-29 million
Primary Classification: The pharmaceutical grade magnesium sulfate market segments by purity level into Purity 99% and Purity>99% categories, representing the evolution from standard pharmaceutical grades to advanced therapeutic formulations for comprehensive medical application optimization.
Secondary Classification: Application segmentation divides the pharmaceutical grade magnesium sulfate market into Pregnancy-Related Diseases, Prevent Hypomagnesemia, Digestive System Diseases, and Others sectors, reflecting distinct requirements for therapeutic protocols, dosing precision, and treatment standards.
Regional Classification: Geographic distribution covers Asia Pacific, Europe, North America, Latin America, and the Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by healthcare infrastructure expansion programs.
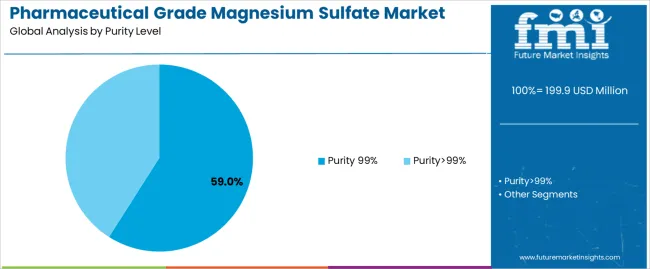
Market Position: Purity>99% grade commands the leading position in the pharmaceutical grade magnesium sulfate market with approximately 59% market share through advanced purification features, including superior therapeutic efficacy, excellent regulatory compliance capability, and quality optimization that enable pharmaceutical manufacturers to achieve optimal therapeutic outcomes across diverse medical treatment environments.
Value Drivers: The segment benefits from hospital pharmacy preference for reliable pharmaceutical compounds that provide consistent therapeutic performance, reduced impurity concerns, and long-term stability optimization without requiring significant protocol modifications. Advanced purification features enable multi-application compatibility, regulatory compliance, and integration with existing pharmaceutical manufacturing equipment, where compound purity and therapeutic reliability represent critical operational requirements.
Competitive Advantages: Purity>99% systems differentiate through proven therapeutic efficacy, consistent pharmaceutical characteristics, and integration with modern quality management processes that enhance operational effectiveness while maintaining optimal safety suitable for diverse medical applications.
Key market characteristics:
Purity 99% formulations maintain approximately 41.0% market share in the pharmaceutical grade magnesium sulfate market due to cost-effectiveness and suitability for non-critical therapeutic applications. These compounds appeal to healthcare facilities requiring standard pharmaceutical-grade materials with acceptable efficacy for general medical applications. Market presence is driven by cost-sensitive procurement, emphasizing adequate therapeutic performance through conventional purification processes.
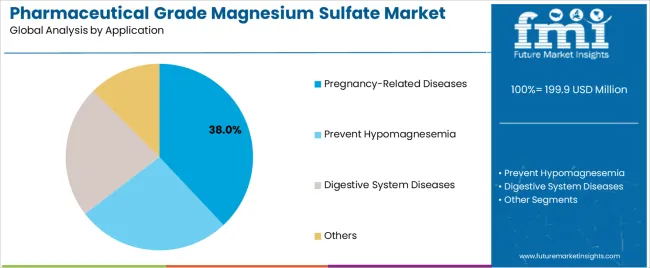
Market Context: Pregnancy-related disease applications dominate the pharmaceutical grade magnesium sulfate market with approximately 38.0% market share due to critical therapeutic requirements in pre-eclampsia and eclampsia management and increasing focus on maternal healthcare optimization, emergency treatment protocols, and obstetric care enhancement applications that minimize pregnancy complications while maintaining maternal-fetal safety standards.
Appeal Factors: Hospital obstetric departments prioritize therapeutic efficacy, safety profiles, and integration with maternal monitoring infrastructure that enables coordinated care across multiple treatment protocols. The segment benefits from substantial maternal healthcare investment and evidence-based medicine programs that emphasize the acquisition of proven therapeutic compounds for critical obstetric applications.
Growth Drivers: Maternal healthcare advancement programs incorporate magnesium sulfate as standard requirements for pre-eclampsia management, while specialty obstetric care increases the need for advanced pharmaceutical-grade formulations that comply with clinical guidelines and minimize adverse outcomes.
Market Challenges: Varying regional obstetric protocols and alternative treatment approaches may limit standardization across different healthcare systems or clinical scenarios.
Application dynamics include:
Hypomagnesemia prevention captures approximately 28.0% market share through electrolyte management requirements in critical care settings, metabolic disorder treatment applications, and nutritional deficiency correction functions. These applications demand pharmaceutical-grade formulations capable of providing precise therapeutic dosing in hospital environments while delivering consistent efficacy and safety characteristics.
Digestive system disease treatments account for approximately 22.0% market share, including laxative applications, gastrointestinal preparation, and constipation management requiring pharmaceutical capabilities for therapeutic effectiveness and patient compliance optimization.
Growth Accelerators: Maternal healthcare advancement drives primary adoption as pharmaceutical grade magnesium sulfate provides critical therapeutic intervention that enables hospitals to meet obstetric care standards for pre-eclampsia management, supporting patient safety and clinical outcome missions that require proven pharmaceutical compounds. Hypomagnesemia recognition accelerates market expansion as healthcare facilities seek pharmaceutical-grade supplements that address electrolyte deficiencies while maintaining therapeutic effectiveness during critical care and metabolic management scenarios. Pharmaceutical quality investment increases worldwide, creating sustained demand for advanced purification systems that complement traditional chemical processes and provide operational flexibility in complex manufacturing environments.
Growth Inhibitors: Raw material cost volatility varies across chemical suppliers regarding magnesium ore sourcing and purification chemicals, which may limit operational flexibility and market penetration in regions with commodity price fluctuations or cost-sensitive pharmaceutical operations. Quality control challenges persist regarding impurity detection and batch consistency that may reduce therapeutic reliability in critical-care temperature, stability, or storage conditions, affecting pharmaceutical performance and safety consistency. Market fragmentation across multiple regulatory standards and pharmacopeia specifications creates compatibility concerns between different supplier capabilities and existing pharmaceutical protocols.
Market Evolution Patterns: Adoption accelerates in maternal healthcare and critical care sectors where therapeutic requirements justify pharmaceutical-grade investments, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by healthcare infrastructure expansion and pharmaceutical manufacturing development. Technology development focuses on enhanced purification methods, improved batch consistency, and integration with automated quality control systems that optimize manufacturing efficiency and regulatory compliance. The pharmaceutical grade magnesium sulfate market could face disruption if alternative magnesium compounds or therapeutic approaches significantly change the deployment of traditional pharmaceutical-grade magnesium sulfate in medical applications.
The pharmaceutical grade magnesium sulfate market demonstrates varied regional dynamics with Growth Leaders including China (5.5% CAGR) and India (5.1% CAGR) driving expansion through healthcare infrastructure development and pharmaceutical manufacturing growth. Steady Performers encompass Germany (4.7% CAGR), Brazil (4.3% CAGR), and United States (3.9% CAGR), benefiting from established pharmaceutical industries and advanced healthcare technology adoption. Mature Markets feature United Kingdom (3.5% CAGR) and Japan (3.1% CAGR), where specialty therapeutic applications and quality-focused procurement support consistent growth patterns.
| Country | CAGR (2025-2035) |
|---|---|
| China | 5.5% |
| India | 5.1% |
| Germany | 4.7% |
| Brazil | 4.3% |
| United States | 3.9% |
| United Kingdom | 3.5% |
| Japan | 3.1% |
Regional synthesis reveals Asia-Pacific markets leading adoption through healthcare system modernization and pharmaceutical manufacturing expansion, while European countries maintain steady growth supported by regulatory advancement and pharmaceutical quality standardization requirements. North American markets show moderate growth driven by maternal healthcare applications and therapeutic protocol integration trends.
How Is Pharmaceutical Manufacturing Expansion Accelerating Market Growth in China?
China establishes high-growth leadership through rapid pharmaceutical manufacturing expansion and comprehensive healthcare infrastructure development, integrating pharmaceutical grade magnesium sulfate as standard materials in hospital pharmacy and therapeutic intervention installations. The country's 5.5% CAGR reflects healthcare modernization initiatives promoting maternal care and pharmaceutical manufacturing capabilities that mandate the use of quality pharmaceutical compounds in hospital and clinical center operations. Growth concentrates in major metropolitan centers, including Shanghai, Beijing, and Guangzhou, where pharmaceutical adoption showcases integrated therapeutic systems that appeal to hospital pharmacies seeking advanced quality standards and regulatory compliance solutions.
Chinese pharmaceutical manufacturers are developing cost-effective production processes that combine competitive pricing advantages with improved quality features, including enhanced purification systems and comprehensive testing protocols. Distribution channels through pharmaceutical distributors and hospital procurement networks expand market access, while quality certification programs for manufacturing facility conversion support adoption across diverse hospital pharmacy and therapeutic application segments.
Strategic Market Indicators:
Why Is Healthcare Infrastructure Development Boosting Market Demand in India?
In Mumbai, Delhi, and Bangalore, hospital pharmacies and pharmaceutical manufacturing facilities are implementing pharmaceutical grade magnesium sulfate as standard materials for therapeutic efficacy and quality assurance, driven by increasing healthcare access and pharmaceutical industry development programs that emphasize the importance of pharmaceutical-grade compounds. The pharmaceutical grade magnesium sulfate market holds a 5.1% CAGR, supported by expanding maternal healthcare programs and pharmaceutical manufacturing advancement initiatives that promote quality compounds for hospital pharmacy and therapeutic facilities. Indian hospitals are adopting Purity>99% formulations that provide therapeutic reliability and regulatory compliance, particularly appealing in urban regions where healthcare quality and pharmaceutical standards represent critical operational requirements.
Market expansion benefits from growing pharmaceutical manufacturing capabilities and technology partnerships that enable domestic production of pharmaceutical-grade compounds for hospital pharmacy and clinical center applications. Technology adoption follows patterns established in pharmaceutical ingredients, where quality and regulatory compliance drive procurement decisions and operational deployment.
Market Intelligence Brief:
How Does Quality Regulation Strengthen Market Leadership in Germany?
Germany's pharmaceutical market demonstrates robust manufacturing capability with documented operational effectiveness in pharmaceutical-grade production and hospital procurement through integration with existing pharmaceutical systems and healthcare infrastructure. The country leverages pharmaceutical manufacturing expertise and regulatory compliance capability to maintain a 4.7% CAGR. Manufacturing facilities, including Stuttgart, Munich, and Frankfurt regions, showcase pharmaceutical installations where high-purity compounds integrate with comprehensive quality management platforms and regulatory systems to optimize pharmaceutical effectiveness.
German pharmaceutical manufacturers prioritize quality assurance and regulatory compliance in compound selection, creating demand for advanced systems with comprehensive testing features, including impurity analysis and batch validation systems. The pharmaceutical-grade magnesium sulfate market benefits from established pharmaceutical infrastructure and willingness to invest in quality-enhancing manufacturing technologies that provide operational benefits and compliance with pharmacopeia requirements.
Market Intelligence Brief:
Why Is Healthcare Modernization Supporting Market Growth in Brazil?
Brazil's market expansion benefits from growing maternal healthcare access, including hospital development in São Paulo and Rio de Janeiro, pharmaceutical manufacturing advancement, and government healthcare programs that increasingly require pharmaceutical-grade compounds for hospital applications. The country maintains a 4.3% CAGR, driven by healthcare infrastructure development and increasing recognition of pharmaceutical quality benefits, including therapeutic reliability and safety optimization capabilities.
Market dynamics focus on cost-effective pharmaceutical solutions that balance quality performance with affordability considerations important to Brazilian healthcare facilities. Growing healthcare awareness creates sustained demand for certified pharmaceutical-grade compounds in hospital infrastructure and healthcare modernization projects.
Strategic Market Considerations:
How Are Hospital Pharmacy Standards Advancing Market Expansion in United States?
The U.S. market emphasizes advanced therapeutic protocols, including evidence-based treatment guidelines and integration with comprehensive hospital pharmacy platforms that handle medication management, quality assurance, and therapeutic monitoring applications through unified healthcare systems. The country holds a 3.9% CAGR, driven by maternal healthcare advancement and hospital pharmacy modernization that support pharmaceutical compound integration. American hospital facilities prioritize therapeutic effectiveness with pharmaceutical compounds delivering consistent clinical outcomes through advanced pharmaceutical quality and safety capabilities.
Technology deployment channels include major hospital networks, specialty obstetric centers, and pharmaceutical procurement programs that support professional applications for complex therapeutic requirements. Hospital pharmacy platform integration capabilities with established management systems expand market appeal across diverse operational requirements seeking efficacy and safety benefits.
Performance Metrics:
How Does Pharmaceutical Quality Compliance Enhance Market Potential in United Kingdom?
In London, Manchester, and pharmaceutical centers, British hospital pharmacies and pharmaceutical manufacturers are implementing pharmaceutical-grade compounds to enhance therapeutic quality capabilities and support modern healthcare operations that align with regulatory standards and quality requirements. The U.K. market holds a 3.5% CAGR, driven by pharmaceutical quality traditions and healthcare efficiency upgrades that emphasize certified compounds for hospital pharmacy and therapeutic applications. British healthcare facilities are prioritizing pharmaceutical-grade formulations that provide regulatory compliance while maintaining therapeutic efficacy, particularly important in specialty care operations and hospital pharmacy facilities.
Market expansion benefits from pharmaceutical quality culture that mandates compound quality capabilities in procurement specifications, creating sustained demand across the UK's hospital pharmacy and healthcare sectors, where pharmaceutical purity and regulatory compliance represent critical requirements. The regulatory framework supports pharmaceutical-grade adoption through quality standards and compliance requirements that promote high-purity compounds aligned with pharmacopeia specifications.
Strategic Market Indicators:
How Are Regulatory Harmonization Programs Shaping Market Development Across Europe?
The European pharmaceutical grade magnesium sulfate market is projected to grow steadily over the forecast period. Germany is expected to maintain its leadership position with a 32.4% market share in 2025, supported by its advanced pharmaceutical manufacturing infrastructure and major hospital networks across Stuttgart, Munich, and Frankfurt regions.
France follows with a 26.8% share in 2025, driven by comprehensive maternal healthcare programs and pharmaceutical quality development initiatives. Spain holds an 18.6% share in 2025 through hospital pharmacy advancement and healthcare system modernization. The United Kingdom commands a 14.2% share, while Italy accounts for 8.0% in 2025. The Rest of Europe region is anticipated to maintain momentum attributed to increasing pharmaceutical manufacturing in Nordic countries and emerging Eastern European healthcare facilities implementing quality pharmaceutical programs.
In Japan, the pharmaceutical grade magnesium sulfate market prioritizes Purity>99% formulations, which capture the dominant share of hospital pharmacy and therapeutic installations due to their advanced features, including superior pharmaceutical quality and seamless integration with existing healthcare infrastructure. Japanese healthcare facilities emphasize quality, regulatory compliance, and therapeutic excellence, creating demand for high-purity compounds that provide consistent pharmaceutical capabilities and adaptive performance based on therapeutic requirements and clinical conditions. Other purity grades maintain secondary positions primarily in specialized applications and cost-sensitive installations where alternative specifications meet operational requirements without compromising therapeutic quality.
Market Characteristics:
In South Korea, the pharmaceutical grade magnesium sulfate market structure favors international pharmaceutical manufacturers, including K+S, PQ Corporation, and Giles, which maintain dominant positions through comprehensive product portfolios and established hospital networks supporting pharmaceutical procurement and therapeutic installations. These providers offer integrated solutions combining pharmaceutical-grade compounds with professional quality assurance services and ongoing technical support that appeal to Korean healthcare facilities seeking reliable pharmaceutical materials. Local distributors and pharmaceutical service providers capture a moderate market share by providing localized technical support and competitive pricing for standard hospital installations, while domestic manufacturers focus on specialized applications and cost-effective solutions tailored to Korean healthcare facility characteristics.
Channel Insights:
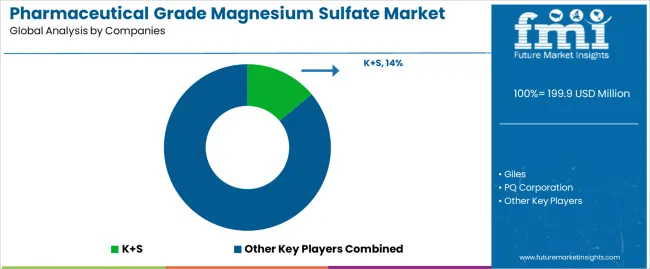
The pharmaceutical grade magnesium sulfate market operates with moderate concentration, featuring approximately 12-15 meaningful participants, where leading companies control roughly 50-55% of the global market share through established pharmaceutical relationships and comprehensive compound portfolios. K+S maintains a leading position with approximately 14.0% market share through extensive pharmaceutical chemical expertise and global distribution operations. Competition emphasizes product purity, regulatory compliance, and technical support rather than price-based rivalry.
Market Leaders encompass K+S, PQ Corporation, and Giles, which maintain competitive advantages through extensive pharmaceutical manufacturing expertise, global pharmaceutical distributor networks, and comprehensive quality assurance capabilities that create customer loyalty and support premium pricing. These companies leverage decades of chemical processing experience and ongoing research investments to develop pharmaceutical-grade compounds with precision purity specifications and regulatory compliance features.
Technology Innovators include Aldeon, UMAI CHEMICAL, and Hebei Meishen Technology, which compete through specialized purification technology focus and innovative quality control interfaces that appeal to pharmaceutical manufacturers seeking advanced pharmaceutical-grade capabilities and regulatory integration. These companies differentiate through rapid quality development cycles and specialized pharmaceutical application focus.
Regional Specialists feature companies like Mani Agro Chem, Gee Gee Kay, and Yingkou Magnesite Chemical, which focus on specific geographic markets and specialized applications, including cost-effective solutions and integrated pharmaceutical systems. Market dynamics favor participants that combine reliable pharmaceutical compounds with advanced technical services, including quality testing support and regulatory documentation capabilities. Competitive pressure intensifies as traditional chemical manufacturers expand into pharmaceutical grades, while specialized pharmaceutical companies challenge established players through innovative purification solutions and quality management platforms targeting modern hospital pharmacy segments.
| Item | Value |
|---|---|
| Quantitative Units | USD 199.9 million |
| Purity Level | Purity 99%, Purity>99% |
| Application | Pregnancy-Related Diseases, Prevent Hypomagnesemia, Digestive System Diseases, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Countries Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 25+ additional countries |
| Key Companies Profiled | K+S, Giles, PQ Corporation, Aldeon, UMAI CHEMICAL, Mani Agro Chem |
| Additional Attributes | Dollar sales by purity level and application categories, regional adoption trends across Asia-Pacific, Europe, and North America, competitive landscape with pharmaceutical manufacturers and chemical suppliers, hospital pharmacy preferences for quality assurance and regulatory compliance, integration with pharmaceutical manufacturing platforms and quality management systems, innovations in purification technologies and pharmaceutical quality standards, and development of certification programs with enhanced technical support and regulatory documentation capabilities. |
The global pharmaceutical grade magnesium sulfate market is estimated to be valued at USD 199.9 million in 2025.
The market size for the pharmaceutical grade magnesium sulfate market is projected to reach USD 298.8 million by 2035.
The pharmaceutical grade magnesium sulfate market is expected to grow at a 4.1% CAGR between 2025 and 2035.
The key product types in pharmaceutical grade magnesium sulfate market are purity 99% and purity>99%.
In terms of application, pregnancy-related diseases segment to command 38.0% share in the pharmaceutical grade magnesium sulfate market in 2025.






Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Pharmaceutical Secondary Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Glass Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Manufacturing Equipment Market Forecast and Outlook 2025 to 2035
Pharmaceutical Plastic Bottle Market Forecast and Outlook 2025 to 2035
Pharmaceutical Industry Analysis in Saudi Arabia Forecast and Outlook 2025 to 2035
Pharmaceutical Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Pots Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Pouch Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Unit Dose Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Mini Batch Blender Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Continuous Manufacturing Equipment Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Liquid Prefilters Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Glass Container Industry Analysis in Europe Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Contract Packaging Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Container Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Sterility Testing Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Preservative Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Track and Trace Systems Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Vials Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA