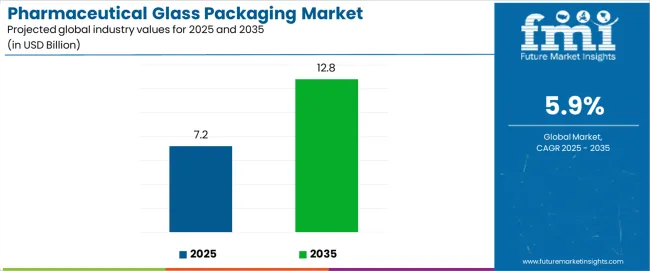
The pharmaceutical glass packaging market stands at the threshold of a decade-long expansion trajectory that promises to reshape drug packaging technology and pharmaceutical delivery solutions. The market's journey from USD 7.2 billion in 2025 to USD 12.8 billion by 2035 represents substantial growth, demonstrating the accelerating adoption of advanced glass packaging technology and premium drug protection solutions across pharmaceutical companies, biopharmaceutical operations, and specialty medicine sectors.
The first half of the decade (2025-2030) will witness the market climbing from USD 7.2 billion to approximately USD 9.4 billion, adding USD 2.2 billion in value, which constitutes 35% of the total forecast growth period. This phase will be characterized by the rapid adoption of premium glass packaging products, driven by increasing biopharmaceutical production and the growing need for advanced sterile packaging solutions worldwide. Enhanced barrier properties and chemical resistance capabilities will become standard expectations rather than premium options.
The latter half (2030-2035) will witness continued growth from USD 9.4 billion to USD 12.8 billion, representing an addition of USD 3.4 billion or 65% of the decade's expansion. This period will be defined by mass market penetration of specialized glass packaging technologies, integration with comprehensive pharmaceutical manufacturing platforms, and seamless compatibility with existing drug production infrastructure. The market trajectory signals fundamental shifts in how pharmaceutical companies approach packaging quality and drug stability management, with participants positioned to benefit from growing demand across multiple container types and therapeutic segments.
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Vials (injectable drugs) | 38% | Vaccines, biologics, sterile solutions |
| Ampoules (single-dose) | 24% | Critical care medicines, oncology drugs | |
| Bottles & containers | 22% | Oral medications, tablets, capsules | |
| Cartridges & syringes | 16% | Pre-filled systems, insulin delivery | |
| Future (3-5 yrs) | Premium borosilicate vials | 32-36% | Biologics growth, high-value therapeutics |
| Pre-filled syringe systems | 22-26% | Patient convenience, self-administration | |
| Specialty ampoules | 18-22% | Oncology expansion, rare disease drugs | |
| Smart glass containers | 12-16% | Digital integration, temperature monitoring | |
| Multi-chamber systems | 8-12% | Combination therapies, lyophilized drugs |
| Metric | Value |
|---|---|
| Market Value (2025) | USD 7.2 billion |
| Market Forecast (2035) | USD 12.8 billion |
| Growth Rate | 5.9% CAGR |
| Leading Technology | Vials |
| Primary Application | Injectable Drugs Segment |
The market demonstrates strong fundamentals with vial systems capturing a dominant share through advanced sterile packaging capabilities and drug stability optimization. Injectable drug applications drive primary demand, supported by increasing biopharmaceutical production and premium glass packaging equipment adoption requirements. Geographic expansion remains concentrated in developed markets with established pharmaceutical manufacturing cultures, while emerging economies show accelerating adoption rates driven by pharmaceutical industry growth and rising quality standards.
Primary Classification: The market segments by container type into vials, ampoules, bottles & containers, and cartridges & syringes, representing the evolution from basic glass packaging materials to sophisticated drug delivery systems for comprehensive pharmaceutical protection optimization.
Secondary Classification: Capacity segmentation divides the market into small containers (up to 10ml), medium containers (10ml-100ml), large containers (100ml-500ml), and extra-large containers (above 500ml), reflecting distinct requirements for drug volume, dosage forms, and therapeutic applications.
Tertiary Classification: Application segmentation covers injectable drugs, oral medications, ophthalmic solutions, topical preparations, and biologics & vaccines, while glass type spans Type I borosilicate glass, Type II treated soda-lime glass, and Type III soda-lime glass categories.
Regional Classification: Geographic distribution covers North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, and Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by pharmaceutical manufacturing expansion programs.
The segmentation structure reveals technology progression from standard glass packaging materials toward sophisticated drug delivery systems with enhanced chemical resistance and barrier properties capabilities, while application diversity spans from generic drug operations to specialized biopharmaceutical establishments requiring precise packaging solutions.
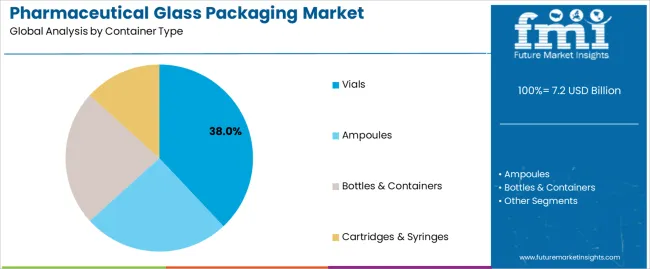
Market Position: Vial systems command the leading position in the pharmaceutical glass packaging market with 38% market share through advanced sterile packaging features, including superior chemical resistance, dimensional precision, and drug stability optimization that enable pharmaceutical companies to achieve optimal product protection across diverse therapeutic and injectable applications.
Value Drivers: The segment benefits from pharmaceutical preference for reliable packaging systems that provide consistent sterile performance, enhanced drug protection, and stability optimization without compromising container integrity or affecting drug efficacy characteristics. Advanced glass engineering enables chemical compatibility, visual inspection capability, and integration with existing filling equipment, where sterile performance and drug stability represent critical regulatory requirements.
Competitive Advantages: Vial systems differentiate through proven chemical resistance, consistent dimensional characteristics, and integration with automated filling systems that enhance manufacturing efficiency while maintaining optimal drug safety standards suitable for diverse injectable and biopharmaceutical applications.
Key market characteristics:
Ampoule systems maintain a 24% market position in the pharmaceutical glass packaging market due to their single-dose convenience and sterile advantages. These systems appeal to pharmaceutical companies requiring tamper-evident packaging with competitive pricing for diverse critical care applications. Market growth is driven by manufacturer preference, emphasizing drug safety and single-use packaging through optimized glass design.
Bottle & container systems capture 22% market share through oral medication requirements in pharmaceutical companies, generic drug manufacturers, and over-the-counter operations. These establishments demand versatile packaging systems capable of handling diverse drug formulations while providing exceptional product protection and regulatory compliance.
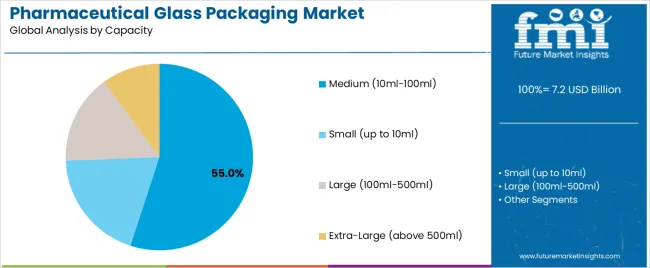
Market Context: Medium capacity containers (10ml-100ml) demonstrate the highest growth rate in the pharmaceutical glass packaging market with 6.8% CAGR due to widespread adoption of multi-dose formulations and increasing focus on biologics packaging, drug consolidation, and manufacturing optimization that maximizes filling efficiency while maintaining sterile standards.
Appeal Factors: Medium container operators prioritize drug volume flexibility, chemical resistance, and integration with diverse therapeutic formulations that enables optimized packaging operations across multiple dosage forms. The segment benefits from substantial biopharmaceutical investment and specialty drug programs that emphasize the acquisition of medium containers for formulation optimization and patient convenience applications.
Growth Drivers: Biopharmaceutical expansion programs incorporate medium containers as standard packaging for multi-dose treatments, while pharmaceutical growth increases demand for versatile capacity capabilities that comply with regulatory standards and minimize packaging complexity.
Market Challenges: Varying drug volumes and formulation requirements may limit container standardization across different therapeutic categories or manufacturing scenarios.
Application dynamics include:
Small capacity container applications capture market share through single-dose requirements in vaccine manufacturing, biologics production, and critical care formulations. These applications demand compact packaging systems capable of operating with sterile filling equipment while providing effective drug protection and regulatory compliance capabilities.
Large capacity container applications account for market share, including bulk pharmaceutical operations, hospital preparations, and high-volume packaging requiring substantial capacity capabilities for manufacturing optimization and cost efficiency.
Market Position: Injectable Drug applications command significant market position with 7.2% CAGR through growing biopharmaceutical production trends and sterile packaging adoption for advanced therapeutic delivery.
Value Drivers: This application segment provides the ideal combination of sterility and chemical resistance, meeting requirements for drug safety, regulatory compliance, and manufacturing efficiency without reliance on alternative packaging materials.
Growth Characteristics: The segment benefits from broad applicability across pharmaceutical companies, automated filling adoption, and established biopharmaceutical programs that support widespread container usage and manufacturing convenience.
Market Context: Biopharmaceutical Companies dominate the market with 7.6% CAGR, reflecting the primary demand source for pharmaceutical glass packaging in biologics production and specialty drug manufacturing platforms.
Business Model Advantages: Biopharmaceutical companies provide direct market demand for high-quality packaging materials, driving innovation standards and manufacturing excellence while maintaining drug safety and regulatory compliance requirements.
Operational Benefits: Biopharmaceutical applications include drug protection, stability assurance, and manufacturing optimization that drive consistent demand for glass containers while providing access to latest packaging technologies.
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Biopharmaceutical growth & biologics expansion (monoclonal antibodies, gene therapies, cell therapies) | ★★★★★ | Exponential growth in biologics creates massive demand for sterile glass packaging; advanced therapies require premium chemical-resistant containers. |
| Driver | Injectable drug proliferation & vaccine production scaling | ★★★★★ | Global vaccination programs and injectable therapeutic growth; standardized sterile packaging requirements across multiple production facilities drive bulk procurement. |
| Driver | Regulatory compliance & quality standards (FDA, EMA, ICH guidelines) | ★★★★★ | Strict pharmaceutical regulations mandate high-quality glass containers; Type I borosilicate glass becomes standard for parenteral formulations. |
| Restraint | Competition from plastic alternatives & advanced polymer systems | ★★★☆☆ | Growing adoption of specialized plastic containers in select applications; cost advantages of polymer systems create substitution pressure. |
| Restraint | Raw material cost volatility & glass manufacturing expenses | ★★★★☆ | Silica sand and energy price fluctuations impact production costs; specialty glass formulations command premium pricing affecting market penetration. |
| Trend | Smart packaging integration & serialization (RFID tags, temperature sensors, anti-counterfeiting) | ★★★★★ | Pharmaceutical companies leveraging packaging for supply chain visibility; digital integration drives premium container adoption. |
| Trend | Pre-filled syringe systems & combination products (auto-injectors, pen systems) | ★★★★☆ | Patient convenience and self-administration trends; integrated delivery systems requiring specialized glass components. |
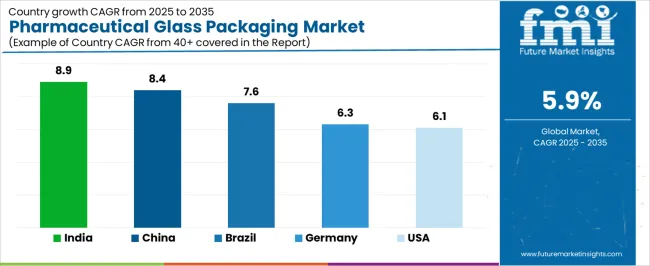
The pharmaceutical glass packaging market demonstrates varied regional dynamics with Growth Leaders including China (8.4% growth rate) and India (8.9% growth rate) driving expansion through pharmaceutical manufacturing growth and generic drug production initiatives. Steady Performers encompass United States (6.1% growth rate), Germany (6.3% growth rate), and developed regions, benefiting from established biopharmaceutical industries and advanced manufacturing adoption. Emerging Markets feature Brazil (7.6% growth rate) and developing regions, where pharmaceutical industry expansion and quality upgrade programs support consistent growth patterns.
Regional synthesis reveals North American and European markets leading value generation through premium product adoption and biopharmaceutical manufacturing culture, while Asian markets demonstrate highest volume growth supported by pharmaceutical production scaling and rising quality standards. European markets show strong growth driven by regulatory packaging requirements and innovation integration.
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| China | 8.4% | Generic drug scale; cost-effective solutions | Quality standardization; regulatory alignment |
| India | 8.9% | Manufacturing expansion; affordable premium containers | Infrastructure development; export compliance |
| United States | 6.1% | Biopharmaceutical innovation; specialized packaging | Regulatory complexity; cost pressures |
| Germany | 6.3% | Quality leadership; advanced manufacturing | Competition intensity; raw material costs |
| Brazil | 7.6% | Local production growth; regional partnerships | Economic volatility; import dependencies |
China establishes fastest market growth through massive pharmaceutical manufacturing infrastructure and comprehensive generic drug development, integrating advanced pharmaceutical glass packaging as standard components in drug production and sterile manufacturing installations. The country's 8.4% growth rate reflects explosive pharmaceutical industry expansion and domestic manufacturing capacity development that mandates the use of quality glass packaging systems in commercial and export facilities. Growth concentrates in major pharmaceutical hubs, including Jiangsu, Zhejiang, and Shandong provinces, where manufacturing clusters showcase integrated packaging systems that appeal to pharmaceutical companies seeking advanced drug protection capabilities and regulatory compliance applications.
Chinese manufacturers are developing domestically-produced container solutions that combine local production advantages with international quality standards, including enhanced chemical resistance and dimensional precision capabilities. Distribution channels through pharmaceutical supply chains and manufacturing partnerships expand market access, while export requirements support adoption across diverse commercial and specialty segments.
Strategic Market Indicators:
In Mumbai, Hyderabad, and Ahmedabad, pharmaceutical companies and generic drug manufacturers are implementing pharmaceutical glass packaging as standard containers for drug safety and regulatory compliance applications, driven by increasing pharmaceutical investment and export programs that emphasize the importance of packaging quality. The market holds an 8.9% growth rate, supported by pharmaceutical industry expansion and manufacturing modernization programs that promote quality container systems for commercial and export applications. Indian operators are adopting packaging solutions that provide consistent chemical resistance and regulatory compliance features, particularly appealing in pharmaceutical regions where drug quality and export standards represent critical business requirements.
Market expansion benefits from growing pharmaceutical exports and manufacturing scale-up that enable widespread adoption of quality packaging systems for pharmaceutical and biopharmaceutical applications. Technology adoption follows patterns established in pharmaceutical manufacturing, where quality and regulatory compliance drive procurement decisions and operational deployment.
Market Intelligence Brief:
United States establishes market leadership through comprehensive biopharmaceutical programs and advanced pharmaceutical industry development, integrating pharmaceutical glass packaging across drug manufacturing and biopharmaceutical applications. The country's 6.1% growth rate reflects established pharmaceutical manufacturing patterns and mature packaging technology adoption that supports widespread use of premium container systems in pharmaceutical and biopharmaceutical facilities. Growth concentrates in major pharmaceutical clusters, including New Jersey, California, and Massachusetts, where biopharmaceutical culture showcases mature container deployment that appeals to pharmaceutical operators seeking proven drug protection capabilities and regulatory compliance applications.
American pharmaceutical establishments leverage established distribution networks and comprehensive product availability, including specialized glass formulations and sterile processing options that create manufacturing advantages and regulatory compliance benefits. The market benefits from mature biopharmaceutical infrastructure and industry willingness to invest in quality packaging materials that enhance drug safety and manufacturing optimization.
Market Intelligence Brief:
Germany's advanced pharmaceutical manufacturing market demonstrates sophisticated pharmaceutical glass packaging deployment with documented quality effectiveness in biopharmaceutical applications and manufacturing facilities through integration with existing production systems and regulatory infrastructure. The country leverages pharmaceutical excellence and manufacturing standards to maintain a 6.3% growth rate. Pharmaceutical centers, including North Rhine-Westphalia, Bavaria, and Baden-Württemberg, showcase premium installations where glass containers integrate with comprehensive manufacturing platforms and quality systems to optimize drug protection operations and regulatory effectiveness.
German operators prioritize manufacturing excellence and quality standards in container procurement, creating demand for premium products with advanced features, including low-alkali compositions and specialized surface treatments. The market benefits from established pharmaceutical engineering expertise and willingness to invest in premium packaging materials that provide superior performance benefits and adherence to quality standards.
Market Intelligence Brief:
Brazil's market expansion benefits from diverse pharmaceutical demand, including generic drug production in São Paulo and Rio de Janeiro, pharmaceutical manufacturing growth, and rising export requirements that increasingly incorporate glass packaging solutions for drug manufacturing applications. The country maintains a 7.6% growth rate, driven by pharmaceutical industry development and increasing recognition of glass packaging benefits, including improved drug protection and enhanced regulatory compliance.
Market dynamics focus on affordable quality container solutions that balance packaging performance with cost considerations important to Brazilian pharmaceutical operators. Growing pharmaceutical manufacturing proliferation creates continued demand for modern packaging systems in new production infrastructure and pharmaceutical modernization projects.
Strategic Market Considerations:
The European pharmaceutical glass packaging market is projected to grow from USD 2.4 billion in 2025 to USD 4.1 billion by 2035, registering a CAGR of 5.5% over the forecast period. Germany is expected to maintain its leadership position with a 29.2% market share in 2025, supported by its strong pharmaceutical manufacturing base and biopharmaceutical industry concentration.
Switzerland follows with a 18.7% share in 2025, driven by pharmaceutical innovation and specialty drug production. France holds a 16.8% share through established pharmaceutical manufacturing and biologics production. United Kingdom commands a 14.3% share, while Italy accounts for 11.6% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 9.4% to 10.2% by 2035, attributed to increasing pharmaceutical manufacturing in Nordic countries and emerging biopharmaceutical establishments implementing quality packaging programs.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global glass manufacturers | Production capacity, glass formulation, distribution networks | Scale efficiency, technical expertise, broad reach | Innovation speed; local customization |
| Specialty pharmaceutical glass | Advanced formulations; chemical resistance technology; premium designs | Quality leadership; pharmaceutical partnerships; differentiation | Mass market penetration; price competition |
| Regional producers | Local manufacturing, distribution channels, competitive pricing | Market proximity; regulatory understanding; cost advantage | Technology gaps; international expansion |
| Contract manufacturers | Pharmaceutical partnerships, custom solutions, specialized processing | Pharmaceutical expertise; regulatory compliance; custom capabilities | Scale limitations; raw material costs |
| Technology innovators | Glass compositions, coating technology, smart packaging solutions | Technical expertise; patent portfolio; performance advantages | Market scalability; distribution challenges |
| Item | Value |
|---|---|
| Quantitative Units | USD 7.2 billion |
| Container Type | Vials, Ampoules, Bottles & Containers, Cartridges & Syringes |
| Capacity | Small (up to 10ml), Medium (10ml-100ml), Large (100ml-500ml), Extra-Large (above 500ml) |
| Glass Type | Type I Borosilicate Glass, Type II Treated Soda-Lime Glass, Type III Soda-Lime Glass |
| Application | Injectable Drugs, Oral Medications, Ophthalmic Solutions, Topical Preparations, Biologics & Vaccines |
| Regions Covered | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia Pacific, Middle East & Africa |
| Countries Covered | United States, China, Germany, India, Switzerland, Brazil, Canada, Japan, France, United Kingdom, and 30+ additional countries |
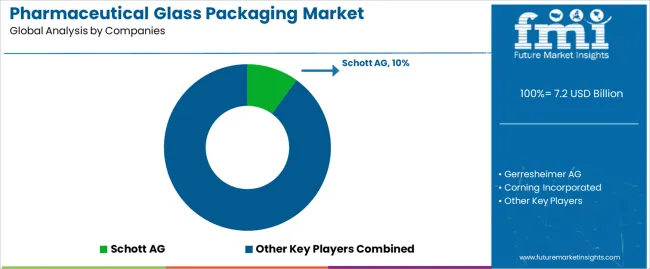
| Key Companies Profiled | Schott AG, Gerresheimer AG, Corning Incorporated, SGD Pharma, Bormioli Pharma, Nipro Corporation, Stevanato Group |
| Additional Attributes | Dollar sales by container type and capacity categories, regional adoption trends across North America, Europe, and East Asia, competitive landscape with glass manufacturers and pharmaceutical suppliers, industry preferences for drug protection and regulatory compliance, integration with filling equipment and manufacturing systems, innovations in chemical resistance technology and dimensional precision, and development of smart packaging solutions with enhanced performance and drug protection capabilities. |
The global pharmaceutical glass packaging market is estimated to be valued at USD 7.2 billion in 2025.
The market size for the pharmaceutical glass packaging market is projected to reach USD 12.8 billion by 2035.
The pharmaceutical glass packaging market is expected to grow at a 5.9% CAGR between 2025 and 2035.
The key product types in pharmaceutical glass packaging market are vials, ampoules, bottles & containers and cartridges & syringes.
In terms of capacity, medium (10ml-100ml) segment to command 55.0% share in the pharmaceutical glass packaging market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Competitive Breakdown of Pharmaceutical Glass Packaging Manufacturers
Pharmaceutical Autoclave Machine Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Excipient SNAC Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Zinc Powder Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade Magnesium Sulfate Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Manufacturing Equipment Market Forecast and Outlook 2025 to 2035
Pharmaceutical Plastic Bottle Market Forecast and Outlook 2025 to 2035
Pharmaceutical Grade Sodium Carbonate Market Forecast and Outlook 2025 to 2035
Pharmaceutical Industry Analysis in Saudi Arabia Forecast and Outlook 2025 to 2035
Pharmaceutical Grade Sodium Chloride Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Plastic Pots Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Pouch Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Mini Batch Blender Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Continuous Manufacturing Equipment Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Liquid Prefilters Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Grade P-Toluenesulfonic Acid Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Container Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Sterility Testing Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceuticals Preservative Market Size and Share Forecast Outlook 2025 to 2035
Pharmaceutical Track and Trace Systems Market Size and Share Forecast Outlook 2025 to 2035

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA