The recombinant human oncostatin M reagent market is valued at USD 116.9 million in 2025 and projected to reach USD 276.8 million by 2035, advancing at a CAGR of 9.0%. The market growth curve indicates a steady rise supported by consistent demand from research laboratories, biotechnology firms, and pharmaceutical developers focusing on cytokine-driven studies. With the increasing role of recombinant proteins in oncology, immunology, and cell biology applications, the adoption rate of oncostatin M reagents has accelerated. Revenue trends suggest that the market will expand at a predictable pace, moving from a mid-size niche in 2025 to a substantially larger segment by 2035, thereby establishing its importance across multiple therapeutic and research domains.
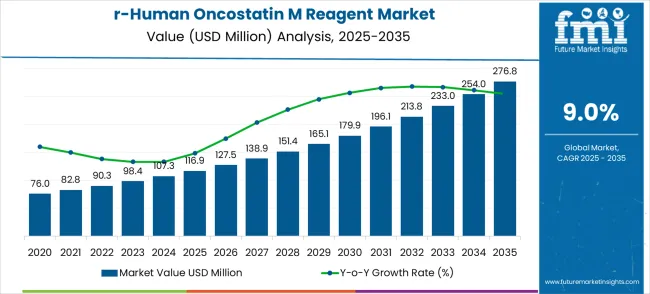
| Metric | Value |
|---|---|
| Market Value (2025) | USD 116.9 million |
| Market Forecast Value (2035) | USD 276.8 million |
| Forecast CAGR (2025-2035) | 9% |
A deeper examination of the growth trajectory highlights clear breakpoints across the timeline. Between 2025 and 2030, the market is expected to show a measured acceleration from USD 116.9 million to nearly USD 165.1 million, supported by greater adoption in cancer biology and tissue regeneration studies. Laboratories are showing preference for high-purity recombinant formulations, which improve experimental consistency and data reproducibility. This initial phase of expansion is expected to be characterized by increasing purchases from research institutions and biopharmaceutical manufacturers, with a particular emphasis on cell-based assay development and preclinical studies.
The second key breakpoint lies between 2030 and 2035, where the market jumps from USD 165.1 million to USD 276.8 million, reflecting faster adoption across applied therapeutic research and diagnostic innovations. During this phase, usage is anticipated to shift beyond traditional laboratory experimentation to more advanced applications in regenerative medicine, chronic disease modeling, and immune response studies. The acceleration is also fueled by growing investments in biologics, with recombinant cytokines such as oncostatin M gaining importance in exploring inflammatory pathways. This phase marks the maturation of the market, transitioning from steady growth to a critical enabler of next-generation biomedical applications.
The recombinant human oncostatin M reagent market is poised for strong growth, driven by biotechnology research expansion, therapeutic development, and precision medicine initiatives. By 2035, these pathways together represent significant incremental revenue opportunities, supporting advanced research and innovation in life sciences.
Pathway A – Advanced Academic Research Applications: Universities and academic research institutions require high-purity cytokine reagents to support immunology, cell biology, and protein interaction studies. Expansion of biotechnology programs and government funding make this a key near-term opportunity, valued at USD 1.0–1.5 billion.
Pathway B – Research Centers & Specialized Labs: Specialized research centers and commercial laboratories demand reliable, reproducible reagents for therapeutic protein development, drug discovery, and complex experimental protocols. This segment represents USD 1.5–2.0 billion in potential revenue.
Pathway C – Precision Medicine & Cytokine Therapy Research: Growing emphasis on precision medicine, regenerative therapies, and cytokine-based therapeutic research drives demand for advanced rhOSM reagents. The expected opportunity pool is USD 0.8–1.2 billion.
Pathway D – Biotech & Pharmaceutical R&D in Emerging Markets: Rapid expansion of biotechnology and pharmaceutical research in China, India, and Latin America creates demand for high-quality recombinant proteins. This pathway offers USD 1.0–1.5 billion incremental revenue.
Pathway E – High-purity Reagent Solutions: Integration of advanced purification technologies and specialized formulations ensures consistent biological activity and experimental reproducibility. This high-purity focus is valued at USD 0.7–1.0 billion.
Pathway F – Regulatory Compliance & Quality Certification: Certification for GMP, ISO, or standardized quality control enhances adoption in research and therapeutic applications. This pathway adds USD 0.5–0.8 billion.
Pathway G – Blended Reagent Systems & Multiplex Applications: Combination with complementary cytokines or protein reagents for advanced assay systems and multiplex studies offers USD 0.4–0.6 billion.
Pathway H – Digital Integration & Laboratory Workflow Optimization: Integration of reagent tracking, digital validation, and workflow management in research laboratories supports adoption of premium rhOSM products. This smaller but strategic pathway contributes USD 0.3–0.5 billion.
Market expansion is being supported by the rapid growth in biotechnology research activities across academic and commercial institutions and the corresponding need for high-quality cytokine reagents that ensure consistent experimental results and research reproducibility. Modern research operations require precise protein reagents with verified biological activity to support advanced studies in immunology, cell biology, and therapeutic development. The superior purity and activity characteristics of recombinant human oncostatin M reagents make them essential components in demanding research environments where experimental accuracy is critical.
The growing emphasis on precision medicine research and therapeutic protein development is driving demand for advanced cytokine reagents from certified manufacturers with proven track records of quality and consistency. Research institutions are increasingly investing in high-purity reagent systems that offer reliable performance and support complex experimental protocols over extended research programs. Regulatory guidelines and research standards are establishing quality benchmarks that favor premium recombinant protein solutions with comprehensive characterization and validation data.
The market is segmented by purity classification, application, and region. By purity classification, the market is divided into Purity<95% and Purity ≥95% configurations. Based on application, the market is categorized into university, research center, and scientists helping scientists. Regionally, the market is divided into North America, Europe, East Asia, South Asia & Pacific, Latin America, and Middle East & Africa.
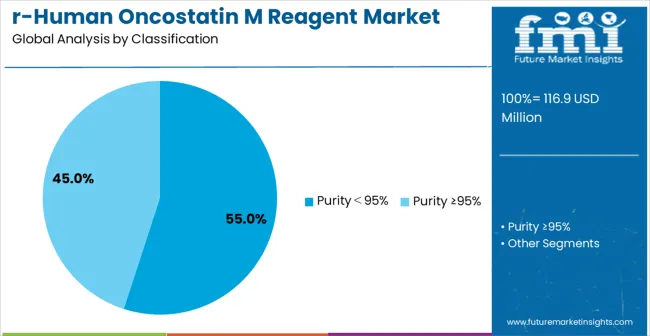
Purity ≥95% reagents are projected to account for the majority of the recombinant human oncostatin M reagent market in 2025. This leading share is supported by the increasing demand for high-purity cytokine reagents in advanced research applications and growing emphasis on experimental reproducibility requiring consistent protein quality. High-purity reagents provide superior biological activity and reliability in critical research protocols, making them the preferred choice for therapeutic research, drug discovery, and advanced biological studies. The segment benefits from technological advancements that have improved purification processes and quality control measures while ensuring consistent batch-to-batch reliability.
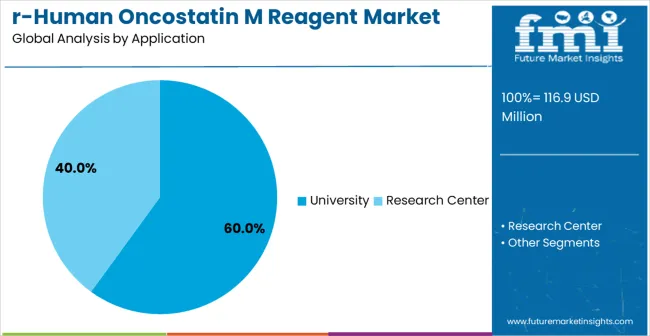
Research center applications are expected to represent the largest share of 60% recombinant human oncostatin M reagent demand in 2025. This dominant position reflects the critical role of specialized research institutions in cytokine studies and their need for highly reliable reagents capable of supporting complex and large-scale experimental protocols. Research centers utilize high-quality cytokine reagents for advanced immunology research, cell biology investigations, therapeutic protein studies, and drug discovery applications, benefiting from their focus on precision research and integration of cutting-edge laboratory technologies.These institutions demand reagents that maintain consistent biological activity, ensure reproducible results, and perform reliably across diverse experimental conditions and extended research programs.
The recombinant human oncostatin M reagent market is advancing rapidly due to increasing biotechnology research activities and growing recognition of cytokine research importance in therapeutic development. However, the market faces challenges including high reagent costs, need for specialized storage conditions, and varying quality requirements across different research applications. Quality standardization efforts and certification programs continue to influence reagent development and market adoption patterns.
The growing deployment of sophisticated purification systems and comprehensive quality testing protocols is enabling enhanced reagent purity and consistent biological activity in recombinant protein production. Advanced chromatography techniques and automated quality control systems provide superior protein characterization while ensuring batch-to-batch consistency and extended shelf life. These technologies are particularly valuable for research institutions that require reliable reagent performance and comprehensive documentation for regulatory compliance.
Modern reagent manufacturers are incorporating advanced formulation technologies and specialized buffer systems that optimize protein stability while enhancing biological activity for specific research applications. Integration of proprietary stabilization methods and application-optimized formulations enables precise performance control and significant improvement in experimental reproducibility compared to standard reagent preparations. Advanced packaging and storage solutions support development of more stable and user-friendly reagent products for demanding research environments.
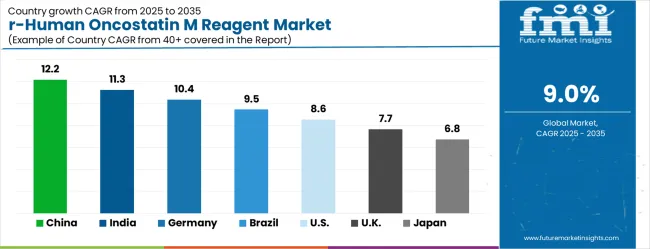
| Country | CAGR (2025-2035) |
|---|---|
| China | 12.2% |
| India | 11.3% |
| Germany | 10.4% |
| Brazil | 9.5% |
| United States | 8.6% |
| United Kingdom | 7.7% |
| Japan | 6.8% |
The recombinant human oncostatin M reagent market is growing rapidly, with China leading at a 12.2% CAGR through 2035, driven by massive biotechnology research expansion, government funding for life sciences, and growing pharmaceutical industry development. India follows at 11.3%, supported by rising academic research activities and increasing investments in biotechnology programs. Germany records strong growth at 10.4%, emphasizing research excellence and advanced biotechnology capabilities. Brazil grows steadily at 9.5%, integrating cytokine research into expanding life sciences programs. The United States shows solid growth at 8.6%, focusing on advanced research applications and therapeutic development. The United Kingdom maintains steady expansion at 7.7%, supported by established research institutions. Japan demonstrates stable growth at 6.8%, emphasizing technological innovation and research quality.
The report covers an in-depth analysis of 40+ countries top-performing countries are highlighted below.
With a projected CAGR of 12.2% through 2035, the recombinant human oncostatin M reagent market in China is leading global expansion, supported by biotechnology research clusters and government-backed funding. Universities, pharmaceutical groups, and academic laboratories are integrating high-purity reagents to strengthen large-scale biological studies. Strong competitive activity is seen as domestic reagent producers attempt to match the quality benchmarks maintained by international suppliers. Collaboration between universities and biotechnology hubs is enhancing procurement channels, enabling efficient access to cytokine reagents for therapeutic and molecular studies. Government incentives are accelerating demand, positioning China as a dominant contributor to the global reagent supply landscape.
Posting a CAGR of 11.3% during 2025–2035, the recombinant human oncostatin M reagent market in India is advancing on the back of academic research growth and biotechnology program expansion. Indian universities and life sciences institutions are increasingly adopting professional-grade reagent systems to achieve reproducibility and consistency in experiments. Competition is gaining intensity with multinational reagent suppliers forming partnerships while domestic players expand regional distribution networks. Biotechnology corridors and government-backed facilities are creating favorable environments for reagent adoption across molecular biology, therapeutic discovery, and cell research. Training programs are building technical expertise within laboratories, ensuring higher reagent utilization and fostering confidence among researchers involved in advanced cytokine-focused projects.
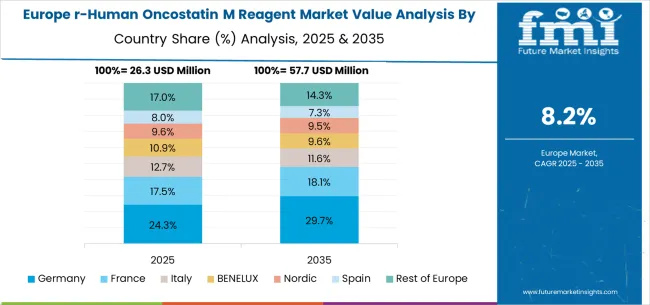
Expected to grow at a CAGR of 10.4% through 2035, the recombinant human oncostatin M reagent market in Germany is strengthened by research excellence initiatives and a focus on biotechnology leadership. German laboratories are adopting reagents that meet high purity standards, ensuring compliance with international research and regulatory frameworks. Market growth is reinforced by pharmaceutical companies and biotechnology firms investing in standardized reagent technologies for advanced drug discovery and clinical studies. Strong rivalry exists between established European suppliers and global reagent manufacturers operating in German clusters. The presence of rigorous training and certification programs has built a skilled workforce, allowing specialized use of cytokine reagents across diverse biological research applications.
At a CAGR of 9.5% from 2025 to 2035, the recombinant human oncostatin M reagent market in Brazil is advancing steadily with increasing life sciences development. Universities, research centers, and biotechnology firms are adopting high-quality reagents to meet evolving experimental requirements. The market benefits from greater international collaboration, enabling access to modern reagent technologies. Local reagent producers are gradually scaling operations, competing with international players who are focused on expanding in Brazil’s biotechnology corridors. Funding directed at academic research is strengthening the demand base, ensuring that research institutions can maintain quality and reproducibility. Workforce enhancement initiatives are providing research personnel with technical know-how to efficiently handle advanced cytokine reagents.
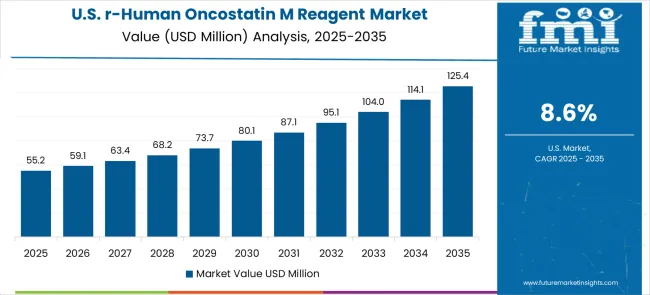
Registering a CAGR of 8.6% between 2025 and 2035, the recombinant human oncostatin M reagent market in the United States is shaped by advanced biotechnology systems and therapeutic development projects. Pharmaceutical giants, biotech companies, and research institutions are incorporating reagent solutions to manage wide-ranging applications from molecular studies to clinical trials. Strong distribution frameworks and high research expenditure provide an advantage to both domestic and multinational suppliers competing in this space. Specialized reagent manufacturers are gaining visibility in niche applications, although large global suppliers dominate mainstream adoption. Workforce development and certification initiatives are ensuring that scientists have the necessary expertise to maximize cytokine reagent effectiveness in therapeutic research.
Forecast at a CAGR of 7.7% until 2035, the recombinant human oncostatin M reagent market in the United Kingdom is shaped by expanding research initiatives and a strong academic base. Universities, pharmaceutical firms, and biotechnology laboratories are adopting reagent systems that comply with rigorous quality and reproducibility standards. Competitive intensity is high as local reagent producers compete with global suppliers within the UK’s well-established research ecosystem. Investments in advanced laboratory infrastructure and training are reinforcing research capacity, allowing the country to strengthen its position as a hub for specialized cytokine-based studies. Professional training programs are ensuring that research personnel can operate high-quality reagents with precision and reliability in diverse applications.
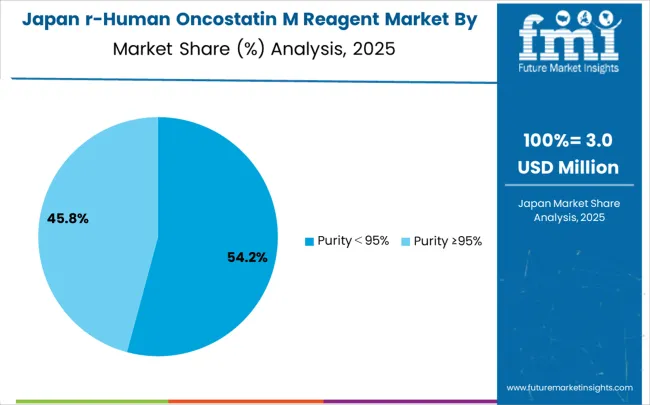
Growing at a CAGR of 6.8% during 2025–2035, the recombinant human oncostatin M reagent market in Japan is supported by a focus on precision biotechnology and quality-driven applications. Japanese research institutions and biotech firms are adopting advanced reagent systems that integrate purification and rigorous quality control. Competition remains strong as global reagent manufacturers target premium market segments, while domestic companies focus on reliability and compatibility with local research protocols. National investments in precision research are broadening reagent usage across molecular biology and therapeutic development programs. Workforce training and certification are equipping researchers with advanced skills, ensuring reliable handling of cytokine reagents across laboratories and specialized biotechnology facilities.
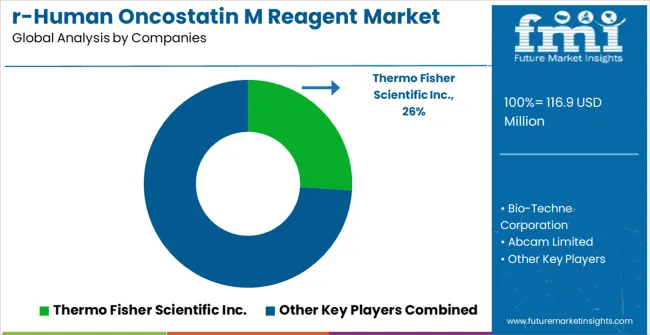
Competition in the recombinant human oncostatin M reagent market is defined by global life sciences leaders, specialized biotechnology companies, and research-focused reagent suppliers. Thermo Fisher Scientific Inc. provides high-purity cytokine reagents with validated biological activity, extensive technical support, and broad compatibility with laboratory workflows. Bio-Techne Corporation emphasizes consistency, reproducibility, and application-specific formulations suitable for both academic and clinical research. Abcam Limited delivers specialized reagents designed for seamless integration into diverse assay platforms with high reliability and robust quality standards.
Bio-Rad Laboratories, Inc., BPS Bioscience, Inc., and Elabscience offer solutions with advanced purification technologies, batch-to-batch consistency, and comprehensive technical documentation. Each company leverages proprietary processes and analytical validation to enhance reagent performance. Sino Biological, Inc., Prospec-Tany Technogene Ltd., and Enzo Biochem, Inc. focus on high-precision production, regulatory compliance, and assay validation to meet demanding research requirements. FUJIFILM Irvine Scientific provides reagents optimized for cell-based assays and broader laboratory applications, while BD Biosciences integrates cytokine products into expansive life sciences portfolios with global distribution and service capabilities.
Product brochures emphasize key attributes such as purity, biological activity, reproducibility, and compatibility with a wide range of experimental assays. Technical specifications, storage stability, and validated performance data are highlighted to demonstrate reliability and operational consistency. Applications across cellular, molecular, and biochemical research are showcased to reinforce scientific value. Strategic partnerships with academic institutions, research centers, and biopharmaceutical companies support innovation and market penetration. Each brochure functions as a direct representation of product quality, technical sophistication, and competitive differentiation, collectively defining the evolving dynamics of the recombinant human oncostatin M reagent market.
| Items | Values |
|---|---|
| Quantitative Units | USD 116.9 million |
| Purity Grade | Purity<95%, Purity ≥95% |
| Application | University, Research Center, Scientists Helping Scientists |
| Regions Covered | North America, Europe, East Asia, South Asia & Pacific, Latin America, Middle East & Africa |
| Country Covered | China, India, Germany, Brazil, United States, United Kingdom, Japan, and 40+ countries |
| Key Companies Profiled | Thermo Fisher Scientific Inc., Bio-Techne Corporation, Abcam Limited, Bio-Rad Laboratories, Inc., BPS Bioscience, Inc., Elabscience, Sino Biological, Inc., Prospec-Tany Technogene Ltd., Enzo Biochem, Inc., FUJIFILM Irvine Scientific, BD Biosciences |
| Additional Attributes | Dollar sales by purity classification and application segment, regional demand trends across major research markets, competitive landscape with established life sciences companies and emerging biotechnology suppliers, customer preferences for high-purity versus standard reagents, integration with research laboratory management systems and experimental protocols, innovations in purification and quality control technologies for enhanced reagent performance, and adoption of specialized formulations with optimized stability and biological activity for improved research outcomes. |
The global recombinant human oncostatin M reagent market is estimated to be valued at USD 116.9 million in 2025.
The market size for the recombinant human oncostatin M reagent market is projected to reach USD 276.8 million by 2035.
The recombinant human oncostatin M reagent market is expected to grow at a 9.0% CAGR between 2025 and 2035.
The key product types in recombinant human oncostatin M reagent market are purity<95% and purity ≥95%.
In terms of application, university segment to command 60.0% share in the recombinant human oncostatin M reagent market in 2025.






Our Research Products

The "Full Research Suite" delivers actionable market intel, deep dives on markets or technologies, so clients act faster, cut risk, and unlock growth.

The Leaderboard benchmarks and ranks top vendors, classifying them as Established Leaders, Leading Challengers, or Disruptors & Challengers.

Locates where complements amplify value and substitutes erode it, forecasting net impact by horizon

We deliver granular, decision-grade intel: market sizing, 5-year forecasts, pricing, adoption, usage, revenue, and operational KPIs—plus competitor tracking, regulation, and value chains—across 60 countries broadly.

Spot the shifts before they hit your P&L. We track inflection points, adoption curves, pricing moves, and ecosystem plays to show where demand is heading, why it is changing, and what to do next across high-growth markets and disruptive tech

Real-time reads of user behavior. We track shifting priorities, perceptions of today’s and next-gen services, and provider experience, then pace how fast tech moves from trial to adoption, blending buyer, consumer, and channel inputs with social signals (#WhySwitch, #UX).

Partner with our analyst team to build a custom report designed around your business priorities. From analysing market trends to assessing competitors or crafting bespoke datasets, we tailor insights to your needs.
Supplier Intelligence
Discovery & Profiling
Capacity & Footprint
Performance & Risk
Compliance & Governance
Commercial Readiness
Who Supplies Whom
Scorecards & Shortlists
Playbooks & Docs
Category Intelligence
Definition & Scope
Demand & Use Cases
Cost Drivers
Market Structure
Supply Chain Map
Trade & Policy
Operating Norms
Deliverables
Buyer Intelligence
Account Basics
Spend & Scope
Procurement Model
Vendor Requirements
Terms & Policies
Entry Strategy
Pain Points & Triggers
Outputs
Pricing Analysis
Benchmarks
Trends
Should-Cost
Indexation
Landed Cost
Commercial Terms
Deliverables
Brand Analysis
Positioning & Value Prop
Share & Presence
Customer Evidence
Go-to-Market
Digital & Reputation
Compliance & Trust
KPIs & Gaps
Outputs
Full Research Suite comprises of:
Market outlook & trends analysis
Interviews & case studies
Strategic recommendations
Vendor profiles & capabilities analysis
5-year forecasts
8 regions and 60+ country-level data splits
Market segment data splits
12 months of continuous data updates
DELIVERED AS:
PDF EXCEL ONLINE
Recombinant DNA Market Size and Share Forecast Outlook 2025 to 2035
Recombinant Vaccines Market Analysis – Trends & Future Outlook 2018-2028
Recombinant Human Serum Albumin Market Size and Share Forecast Outlook 2025 to 2035
Prokaryotic Recombinant Protein Market
Human Torso Simulator Market Size and Share Forecast Outlook 2025 to 2035
Human Transferrin Detection Kit Market Size and Share Forecast Outlook 2025 to 2035
Human Papilloma Virus Testing Market Size and Share Forecast Outlook 2025 to 2035
Human-Centric Lighting Market Size and Share Forecast Outlook 2025 to 2035
Human Identification Market Size and Share Forecast Outlook 2025 to 2035
Human Immunodeficiency Virus Type 1 (HIV 1) Market Size and Share Forecast Outlook 2025 to 2035
Humanoid Robot Market Size and Share Forecast Outlook 2025 to 2035
Humanized Mouse Model Market Size and Share Forecast Outlook 2025 to 2035
Human Milk Oligosaccharides Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Human Combinatorial Antibody Libraries (HuCAL) Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
Human Growth Hormone (HGH) Treatment and Drugs Market Trends - Growth & Forecast 2025 to 2035
Human Augmentation Technology Market Growth - Trends & Forecast 2025 to 2035
Key Companies & Market Share in the Human Milk Oligosaccharides Sector
Human RSV Treatment Market Insights - Innovations & Forecast 2025 to 2035
Human Osteoblasts Market – Growth & Forecast 2024-2034
Human Capital Management Market

Thank you!
You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Chat With
MaRIA